Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety
- PMID: 24241397
- PMCID: PMC4035371
- DOI: 10.1038/nn.3582
Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety
Abstract
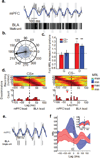
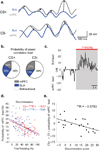
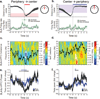
Successfully differentiating safety from danger is an essential skill for survival. While decreased activity in the medial prefrontal cortex (mPFC) is associated with fear generalization in animals and humans, the circuit-level mechanisms used by the mPFC to discern safety are not clear. To answer this question, we recorded activity in the mPFC, basolateral amygdala (BLA) and dorsal and ventral hippocampus in mice during exposure to learned (differential fear conditioning) and innate (open field) anxiety. We found increased synchrony between the mPFC and BLA in the theta frequency range (4-12 Hz) only in animals that differentiated between averseness and safety. Moreover, during recognized safety across learned and innate protocols, BLA firing became entrained to theta input from the mPFC. These data suggest that selective tuning of BLA firing to mPFC input provides a safety-signaling mechanism whereby the mPFC taps into the microcircuitry of the amygdala to diminish fear.
Figures





References
-
- Resnik J, Sobel N, Paz R. Auditory aversive learning increases discrimination thresholds. Nat. Neurosci. 2011;14:791–796. - PubMed
-
- Reinecke A, Becker ES, Hoyer J, Rinck M. Generalized implicit fear associations in generalized anxiety disorder. Depress. Anxiety. 2010;27:252–259. - PubMed
-
- Gazendam FJ, Kamphuis JH, Kindt M. Deficient safety learning characterizes high trait anxiety individuals. Biol Psychol. 2013;92:342–352. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

