Imatinib adherence associated clinical outcomes of chronic myeloid leukaemia treatment in Taiwan
- PMID: 24242992
- PMCID: PMC3890042
- DOI: 10.1007/s11096-013-9876-7
Imatinib adherence associated clinical outcomes of chronic myeloid leukaemia treatment in Taiwan
Abstract
Background: Since the launch of imatinib, chronic myeloid leukaemia has become a chronic condition requiring costly long-term treatment. Emerging evidence from several short-term studies has raised concerns on the detrimental clinical outcomes and waste of resources associated with poor adherence to imatinib.
Objective: This study aims to evaluate the effects of long-term imatinib adherence on clinical treatment responses and mortality.
Setting: This retrospective cohort study was conducted in a medical centre in southern Taiwan.
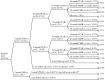
Method: Chronic myeloid leukaemia patients who were prescribed for more than 1 month of imatinib were identified and their medical charts were reviewed from the first date of imatinib prescription to the last date of medical record or upon patients' death. Patients' basic characteristics, imatinib prescriptions, results of laboratory tests, episodes of imatinib-related side effects and mortality rate were recorded.
Main outcome measure: Participants' basic characteristics, medication possession ratio and their mortality rate; the association between the medication possession ratio and treatment responses.
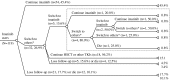
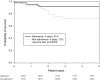
Results: Of the 119 included patients, the mean follow-up time was 3.9 ± 2.9 patient-years and the mean medication possession ratio was 89.7 %. At the 18th month of imatinib treatment, 67.2, 54.3 and 34.5 % patients achieved complete cytogenetic, major molecular and complete molecular responses, respectively. There was a significant difference in the 4-year survival rate between the adherence (n = 87) and non-adherence (n = 32) groups (91 vs. 72 %; p = 0.0076). Logistic regression analysis revealed that imatinib adherence was the only factor that significantly influenced the 18th month complete cytogenetic response [odds ratio (OR) 11.6; 95 % confidence interval (CI) 1.7, 114.7; p = 0.0131] and major molecular response (OR 5.1; 95 % CI 1.1, 26.8; p = 0.0351). Cox regression analysis demonstrated that a medication possession ratio greater than 90 % significantly reduced the mortality risk (hazard ratio 0.1; 95 % CI 0.01, 0.60; p = 0.0118).
Conclusion: Chronic myeloid leukaemia patients' long-term adherence to imatinib is significantly associated with the 18th month treatment responses including the cytogenetic response, molecular response and the long-term survival rate in clinical practice.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

