Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys
- PMID: 24243013
- PMCID: PMC3846288
- DOI: 10.1016/j.cell.2013.09.061
Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys
Abstract
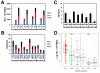
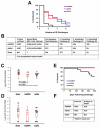
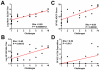
The global diversity of HIV-1 represents a critical challenge facing HIV-1 vaccine development. HIV-1 mosaic antigens are bioinformatically optimized immunogens designed for improved coverage of HIV-1 diversity. However, the protective efficacy of such global HIV-1 vaccine antigens has not previously been evaluated. Here, we demonstrate the capacity of bivalent HIV-1 mosaic antigens to protect rhesus monkeys against acquisition of infection following heterologous challenges with the difficult-to-neutralize simian-human immunodeficiency virus SHIV-SF162P3. Adenovirus/poxvirus and adenovirus/adenovirus vector-based vaccines expressing HIV-1 mosaic Env, Gag, and Pol afforded a significant reduction in the per-exposure acquisition risk following repetitive, intrarectal SHIV-SF162P3 challenges. Protection against acquisition of infection correlated with vaccine-elicited binding, neutralizing, and functional nonneutralizing antibodies, suggesting that the coordinated activity of multiple antibody functions may contribute to protection against difficult-to-neutralize viruses. These data demonstrate the protective efficacy of HIV-1 mosaic antigens and suggest a potential strategy for the development of a global HIV-1 vaccine. PAPERCLIP:
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Comment in
-
First steps toward a globally effective HIV/AIDS vaccine.Cell. 2013 Oct 24;155(3):495-7. doi: 10.1016/j.cell.2013.10.012. Epub 2013 Oct 24. Cell. 2013. PMID: 24243006
-
HIV: advancing the antibody approach.Nat Rev Immunol. 2013 Dec;13(12):846-7. doi: 10.1038/nri3573. Nat Rev Immunol. 2013. PMID: 24409525 No abstract available.
References
-
- Barnett SW, Burke B, Sun Y, Kan E, Legg H, Lian Y, Bost K, Zhou F, Goodsell A, Zur Megede J, et al. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J Virol. 2010;84:5975–5985. - PMC - PubMed
-
- Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, Cristillo AD, Ferrai MG, Weiss DE, Letvin NL, Montefiori D, et al. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. Aids. 2008;22:339–348. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- T32 AI007387/AI/NIAID NIH HHS/United States
- U19 AI078526/AI/NIAID NIH HHS/United States
- UM1 AI100645/AI/NIAID NIH HHS/United States
- AI096040/AI/NIAID NIH HHS/United States
- T32 AI052074/AI/NIAID NIH HHS/United States
- AI100645/AI/NIAID NIH HHS/United States
- R01 AI084794/AI/NIAID NIH HHS/United States
- AI078526/AI/NIAID NIH HHS/United States
- AI060354/AI/NIAID NIH HHS/United States
- U19 AI096040/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- P40 OD012217/OD/NIH HHS/United States
- UM1 AI100663/AI/NIAID NIH HHS/United States
- AI095985/AI/NIAID NIH HHS/United States
- AI084794/AI/NIAID NIH HHS/United States
- U19 AI095985/AI/NIAID NIH HHS/United States
- AI052074/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

