A comparison of replicative senescence and doxorubicin-induced premature senescence of vascular smooth muscle cells isolated from human aorta
- PMID: 24243065
- PMCID: PMC3905196
- DOI: 10.1007/s10522-013-9477-9
A comparison of replicative senescence and doxorubicin-induced premature senescence of vascular smooth muscle cells isolated from human aorta
Abstract
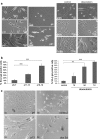
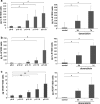
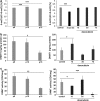
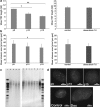
Senescence of vascular smooth muscle cells (VSMCs) contributes to aging as well as age-related diseases of the cardiovascular system. Senescent VSMCs have been shown to be present in atherosclerotic plaques. Both replicative (RS) and stress-induced premature senescence (SIPS) accompany cardiovascular diseases. We aimed to establish the signature of RS and SIPS of VSMCs, induced by a common anticancer drug, doxorubicin, and to discover the so far undisclosed features of senescent cells that are potentially harmful to the organism. Most of the senescence hallmarks were common for both RS and SIPS; however, some differences were observed. 32 % of doxorubicin-treated cells were arrested in the G2/M phase of the cell cycle, while 73 % of replicatively senescing cells were arrested in the G1 phase. Moreover, on the basis of alkaline phosphatase activity measurements, we show that a 7-day treatment with doxorubicin (dox), does not cause precocious cell calcification, which is a characteristic feature of RS. We did not observe calcification even though after 7 days of dox-treatment many other markers characteristic for senescent cells were present. It can suggest that dox-induced SIPS does not accelerate the mineralization of vessels. We consider that detailed characterization of the two types of cellular senescence can be useful in in vitro studies of potential anti-aging factors.
Figures




References
-
- Bennett MR, Macdonald K, Chan SW, Boyle JJ, Weissberg PL. Cooperative interactions between RB and p53 regulate cell proliferation, cell senescence, and apoptosis in human vascular smooth muscle cells from atherosclerotic plaques. Circ Res. 1998;82:704–712. doi: 10.1161/01.RES.82.6.704. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

