Enhancing protein disaggregation restores proteasome activity in aged cells
- PMID: 24243762
- PMCID: PMC3868723
- DOI: 10.18632/aging.100613
Enhancing protein disaggregation restores proteasome activity in aged cells
Abstract
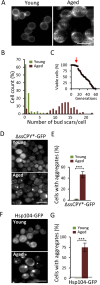
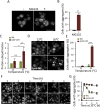
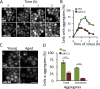
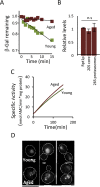
The activity of the ubiquitin-proteasome system, UPS, declines during aging in several multicellular organisms. The reason behind this decline remains elusive. Here, using yeast as a model system, we show that while the level and potential capacity of the 26S proteasome is maintained in replicatively aged cells, the UPS is not functioning properlyin vivo. As a consequence cytosolic UPS substrates, such as ΔssCPY* are stabilized, accumulate, and form inclusions. By integrating a pGPD-HSP104 recombinant gene into the genome, we were able to constitutively elevate protein disaggregase activity, which diminished the accumulation of protein inclusions during aging. Remarkably, this elevated disaggregation restored degradation of a 26S proteasome substrate in aged cells without elevating proteasome levels, demonstrating that age-associated aggregation obstructs UPS function. The data supports the existence of a negative feedback loop that accelerates aging by exacerbating proteostatic decline once misfolded and aggregation-prone proteins reach a critical level.
Conflict of interest statement
The authors of this manuscript declare no conflicts of interest.
Figures

References
-
- Stadtman ER, Starke-Reed PE, Oliver CN, Carney JM, Floyd RA. Protein modification in aging. Exs. 1992;62:64–72. - PubMed
-
- Barja G. Mitochondrial free radical production and aging in mammals and birds. Annals of the New York Academy of Sciences. 1998;854:224–238. - PubMed
-
- Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free radical biology & medicine. 2002;32:790–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

