Cutting edge: Leukotriene C4 activates mouse platelets in plasma exclusively through the type 2 cysteinyl leukotriene receptor
- PMID: 24244016
- PMCID: PMC3869987
- DOI: 10.4049/jimmunol.1302187
Cutting edge: Leukotriene C4 activates mouse platelets in plasma exclusively through the type 2 cysteinyl leukotriene receptor
Abstract
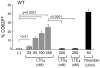
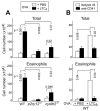
Leukotriene C4 (LTC4) and its extracellular metabolites, LTD4 and LTE4, mediate airway inflammation. They signal through three specific receptors (type 1 cys-LT receptor [CysLT1R], CysLT2R, and GPR99) with overlapping ligand preferences. In this article, we demonstrate that LTC4, but not LTD4 or LTE4, activates mouse platelets exclusively through CysLT2R. Platelets expressed CysLT1R and CysLT2R proteins. LTC4 induced surface expression of CD62P by wild-type mouse platelets in platelet-rich plasma (PRP) and caused their secretion of thromboxane A2 and CXCL4. LTC4 was fully active on PRP from mice lacking either CysLT1R or GPR99, but completely inactive on PRP from CysLT2R-null (Cysltr2(-/-)) mice. LTC4/CysLT2R signaling required an autocrine ADP-mediated response through P2Y12 receptors. LTC4 potentiated airway inflammation in a platelet- and CysLT2R-dependent manner. Thus, CysLT2R on platelets recognizes LTC4 with unexpected selectivity. Nascent LTC4 may activate platelets at a synapse with granulocytes before it is converted to LTD4, promoting mediator generation and the formation of leukocyte-platelet complexes that facilitate inflammation.
Figures


References
-
- Drazen JM, Israel E. Treatment of chronic stable asthma with drugs active on the 5-lipoxygenase pathway. Int Arch Allergy Immunol. 1995;107:319–320. - PubMed
-
- Dixon RA, Diehl RE, Opas E, Rands E, Vickers PJ, Evans JF, Gillard JW, Miller DK. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL117945/HL/NHLBI NIH HHS/United States
- T32 AI007306/AI/NIAID NIH HHS/United States
- U19 AI095219/AI/NIAID NIH HHS/United States
- K23 HL111113/HL/NHLBI NIH HHS/United States
- R21 AI082369/AI/NIAID NIH HHS/United States
- HL111113/HL/NHLBI NIH HHS/United States
- AI095219/AI/NIAID NIH HHS/United States
- AT002782/AT/NCCIH NIH HHS/United States
- P50 AT002782/AT/NCCIH NIH HHS/United States
- R01 AI052353/AI/NIAID NIH HHS/United States
- AI078908/AI/NIAID NIH HHS/United States
- P01 HL036110/HL/NHLBI NIH HHS/United States
- AI082369/AI/NIAID NIH HHS/United States
- R01 AI078908/AI/NIAID NIH HHS/United States
- HL36110/HL/NHLBI NIH HHS/United States
- U01 HL102225/HL/NHLBI NIH HHS/United States
- R37 AI052353/AI/NIAID NIH HHS/United States
- HL117945/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

