Optineurin insufficiency impairs IRF3 but not NF-κB activation in immune cells
- PMID: 24244017
- PMCID: PMC3886234
- DOI: 10.4049/jimmunol.1301696
Optineurin insufficiency impairs IRF3 but not NF-κB activation in immune cells
Abstract
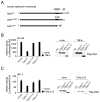
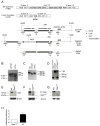
Optineurin is a widely expressed polyubiquitin-binding protein that has been implicated in regulating cell signaling via its NF-κB essential modulator-homologous C-terminal ubiquitin (Ub)-binding region. Its functions are controversial, with in vitro studies finding that optineurin suppressed TNF-mediated NF-κB activation and virus-induced activation of IFN regulatory factor 3 (IRF3), whereas bone marrow-derived macrophages (BMDMs) from mice carrying an optineurin Ub-binding point mutation had normal TLR-mediated NF-κB activation and diminished IRF3 activation. We have generated a mouse model in which the entire Ub-binding C-terminal region is deleted (Optn(470T)). Akin to C-terminal optineurin mutations found in patients with certain neurodegenerative diseases, Optn(470T) was expressed at substantially lower levels than the native protein, allowing assessment not only of the lack of Ub binding, but also of protein insufficiency. Embryonic lethality with incomplete penetrance was observed for 129 × C57BL/6 Optn(470T/470T) mice, but after further backcrossing to C57BL/6, offspring viability was restored. Moreover, the mice that survived were indistinguishable from wild type littermates and had normal immune cell distributions. Activation of NF-κB in Optn(470T) BMDM and BM-derived dendritic cells with TNF or via TLR4, T cells via the TCR, and B cells with LPS or anti-CD40 was normal. In contrast, optineurin and/or its Ub-binding function was necessary for optimal TANK binding kinase 1 and IRF3 activation, and both Optn(470T) BMDMs and bone marrow-derived dendritic cells had diminished IFN-β production upon LPS stimulation. Importantly, Optn(470T) mice produced less IFN-β upon LPS challenge. Therefore, endogenous optineurin is dispensable for NF-κB activation but necessary for optimal IRF3 activation in immune cells.
Figures



References
-
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

