A phase 1b randomized, controlled, double-blinded dosage-escalation trial to evaluate the safety, reactogenicity and immunogenicity of an adenovirus type 35 based circumsporozoite malaria vaccine in Burkinabe healthy adults 18 to 45 years of age
- PMID: 24244339
- PMCID: PMC3823848
- DOI: 10.1371/journal.pone.0078679
A phase 1b randomized, controlled, double-blinded dosage-escalation trial to evaluate the safety, reactogenicity and immunogenicity of an adenovirus type 35 based circumsporozoite malaria vaccine in Burkinabe healthy adults 18 to 45 years of age
Abstract
Background: Ad35.CS.01 is a pre-erythrocytic malaria candidate vaccine. It is a codon optimized nucleotide sequence representing the P. falciparum circumsporozoite (CS) surface antigen inserted in a replication deficient Adenovirus 35 backbone. A Phase 1a trial has been conducted in the USA in naïve adults and showed that the vaccine was safe. The aim of this study is to assess the safety and immunogenicity of ascending dosages in sub Saharan Africa.
Methods: A double blind, randomized, controlled, dose escalation, phase Ib trial was conducted in a rural area of Balonghin, the Saponé health district (Burkina Faso). Forty-eight healthy adults aged 18-45 years were randomized into 4 cohorts of 12 to receive three vaccine doses (day 0, 28 and 84) of 10(9), 10(10), 5X10(10), 10(11) vp of Ad35.CS.01 or normal saline by intra muscular injection. Subjects were monitored carefully during the 14 days following each vaccination for non serious adverse events. Severe and serious adverse events were collected throughout the participant study duration (12 months from the first vaccination). Humoral and cellular immune responses were measured on study days 0, 28, 56, 84, 112 and 140.
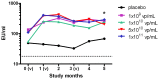
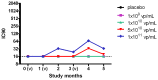
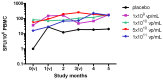
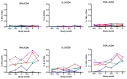
Results: Of the forty-eight subjects enrolled, forty-four (91.7%) received all three scheduled vaccine doses. Local reactions, all of mild severity, occurred in thirteen (27.1%) subjects. Severe (grade 3) laboratory abnormalities occurred in five (10.4%) subjects. One serious adverse event was reported and attributed to infection judged unrelated to vaccine. The vaccine induced both antibody titers and CD8 T cells producing IFNγ and TNFα with specificity to CS while eliciting modest neutralizing antibody responses against Ad35.
Conclusion: Study vaccine Ad35.CS.01 at four different dose levels was well-tolerated and modestly immunogenic in this population. These results suggest that Ad35.CS.01 should be further investigated for preliminary efficacy in human challenge models and as part of heterologous prime-boost vaccination strategies.
Trial registration: ClinicalTrials.gov NCT01018459 http://clinicaltrials.gov/ct2/show/NCT01018459.
Conflict of interest statement
Figures
References
-
- World Health Organization (2011). Available: http://www.who.int/malaria/publications/atoz/9789241564403/en/index.html. Accessed 2010 June 1.
-
- Gantt SM, Clavijo P, Bai X, Esko JD, Sinnis P (1997) Cell adhesion to a motif shared by the malaria circumsporozoite protein and thrombospondin is mediated by its glycosaminoglycan-binding region and not by CSVTCG. J Biol Chem 272: 19205-19213. doi:10.1074/jbc.272.31.19205. PubMed: 9235912. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

