A novel nonhuman primate model for influenza transmission
- PMID: 24244352
- PMCID: PMC3828296
- DOI: 10.1371/journal.pone.0078750
A novel nonhuman primate model for influenza transmission
Abstract
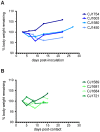
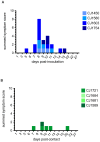
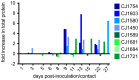
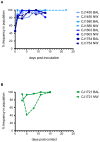
Studies of influenza transmission are necessary to predict the pandemic potential of emerging influenza viruses. Currently, both ferrets and guinea pigs are used in such studies, but these species are distantly related to humans. Nonhuman primates (NHP) share a close phylogenetic relationship with humans and may provide an enhanced means to model the virological and immunological events in influenza virus transmission. Here, for the first time, it was demonstrated that a human influenza virus isolate can productively infect and be transmitted between common marmosets (Callithrix jacchus), a New World monkey species. We inoculated four marmosets with the 2009 pandemic virus A/California/07/2009 (H1N1pdm) and housed each together with a naïve cage mate. We collected bronchoalveolar lavage and nasal wash samples from all animals at regular intervals for three weeks post-inoculation to track virus replication and sequence evolution. The unadapted 2009 H1N1pdm virus replicated to high titers in all four index animals by 1 day post-infection. Infected animals seroconverted and presented human-like symptoms including sneezing, nasal discharge, labored breathing, and lung damage. Transmission occurred in one cohabitating pair. Deep sequencing detected relatively few genetic changes in H1N1pdm viruses replicating in any infected animal. Together our data suggest that human H1N1pdm viruses require little adaptation to replicate and cause disease in marmosets, and that these viruses can be transmitted between animals. Marmosets may therefore be a viable model for studying influenza virus transmission.
Conflict of interest statement
Figures

References
-
- Bodewes R, Rimmelzwaan GF, Osterhaus AD (2010) Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev Vaccines 9: 59–72. - PubMed
-
- Mansfield K (2003) Marmoset models commonly used in biomedical research. Comp Med 53: 383–392. - PubMed
-
- United States National Research Council (1996) Guide for the care and use of laboratory animals.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

