Arabidopsis AtRRP44A is the functional homolog of Rrp44/Dis3, an exosome component, is essential for viability and is required for RNA processing and degradation
- PMID: 24244451
- PMCID: PMC3820695
- DOI: 10.1371/journal.pone.0079219
Arabidopsis AtRRP44A is the functional homolog of Rrp44/Dis3, an exosome component, is essential for viability and is required for RNA processing and degradation
Abstract
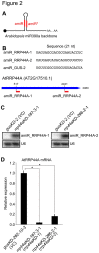
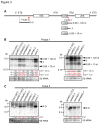
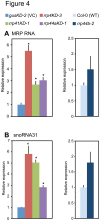
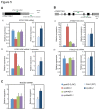
The RNA exosome is a multi-subunit complex that is responsible for 3' to 5' degradation and processing of cellular RNA. Rrp44/Dis3 is the catalytic center of the exosome in yeast and humans. However, the role of Rrp44/Dis3 homologs in plants is still unidentified. Here, we show that Arabidopsis AtRRP44A is the functional homolog of Rrp44/Dis3, is essential for plant viability and is required for RNA processing and degradation. We characterized AtRRP44A and AtRRP44B/SOV, two predicted Arabidopsis Rrp44/Dis3 homologs. AtRRP44A could functionally replace S. cerevisiae Rrp44/Dis3, but AtRRP44B/SOV could not. rrp44a knock-down mutants showed typical phenotypes of exosome function deficiency, 5.8S rRNA 3' extension and rRNA maturation by-product over-accumulation, but rrp44b mutants did not. Conversely, AtRRP44B/SOV mutants showed elevated levels of a selected mRNA, on which rrp44a did not have detectable effects. Although T-DNA insertion mutants of AtRRP44B/SOV had no obvious phenotype, those of AtRRP44A showed defects in female gametophyte development and early embryogenesis. These results indicate that AtRRP44A and AtRRP44B/SOV have independent roles for RNA turnover in plants.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

