KSRP modulation of GAP-43 mRNA stability restricts axonal outgrowth in embryonic hippocampal neurons
- PMID: 24244461
- PMCID: PMC3828348
- DOI: 10.1371/journal.pone.0079255
KSRP modulation of GAP-43 mRNA stability restricts axonal outgrowth in embryonic hippocampal neurons
Abstract
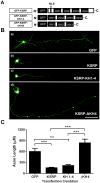
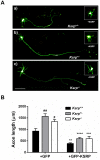
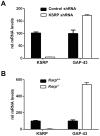
The KH-type splicing regulatory protein (KSRP) promotes the decay of AU-rich element (ARE)-containing mRNAs. Although KSRP is expressed in the nervous system, very little is known about its role in neurons. In this study, we examined whether KSRP regulates the stability of the ARE-containing GAP-43 mRNA. We found that KSRP destabilizes this mRNA by binding to its ARE, a process that requires the presence of its fourth KH domain (KH4). Furthermore, KSRP competed with the stabilizing factor HuD for binding to these sequences. We also examined the functional consequences of KSRP overexpression and knockdown on the differentiation of primary hippocampal neurons in culture. Overexpression of full length KSRP or KSRP without its nuclear localization signal hindered axonal outgrowth in these cultures, while overexpression of a mutant protein without the KH4 domain that has less affinity for binding to GAP-43's ARE had no effect. In contrast, depletion of KSRP led to a rise in GAP-43 mRNA levels and a dramatic increase in axonal length, both in KSRP shRNA transfected cells and neurons cultured from Ksrp(+/-) and Ksrp(-/-) embryos. Finally we found that overexpression of GAP-43 rescued the axonal outgrowth deficits seen with KSRP overexpression, but only when cells were transfected with GAP-43 constructs containing 3' UTR sequences targeting the transport of this mRNA to axons. Together, our results suggest that KSRP is an important regulator of mRNA stability and axonal length that works in direct opposition to HuD to regulate the levels of GAP-43 and other ARE-containing neuronal mRNAs.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

