Neuropsychological deficits in mice depleted of the schizophrenia susceptibility gene CSMD1
- PMID: 24244513
- PMCID: PMC3828352
- DOI: 10.1371/journal.pone.0079501
Neuropsychological deficits in mice depleted of the schizophrenia susceptibility gene CSMD1
Abstract
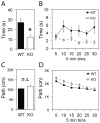
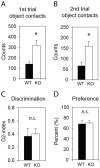
Recent meta-analyses of schizophrenia genome-wide association studies (GWASs) have identified the CUB and SUSHI multiple domains 1 (CSMD1) gene as a statistically strong risk factor. CSMD1 is a complement control-related protein suggested to inhibit the classical complement pathway, being expressed in developing neurons. However, expression of CSMD1 is largely uncharacterized and relevance for behavioral phenotypes is not previously demonstrated. Here, we assess neuropsychological behaviors of a Csmd1 knockout (KO) mouse in a selection of standard behavioral tests. Deregulation of neuropsychological responses were observed in both the open field and the elevated plus maze tests, in which KO mice spent 55% and 33% less time than WT littermate mice in open areas, respectively. Altered behaviors were also observed in tail suspension and to higher acoustic stimuli, for which Csmd1 KO mice showed helplessness and moderate increase in startle amplitude, respectively. Furthermore, Csmd1 KO mice also displayed increased weight-gain and glucose tolerance, similar to a major phenotype of the metabolic syndrome that also has been associated to the human CSMD1 locus. Consistent with a role in the control of behaviors, Csmd1 was found highly expressed in the central nervous system (CNS), and with some expression in visceral fat and ovary, under tissue-specific control by a novel promoter-associated lncRNA. In summary, disruption of Csmd1 induces behaviors reminiscent of blunted emotional responses, anxiety and depression. These observations suggest an influence of the CSMD1 schizophrenia susceptibility gene on psychopathology and endophenotypes of the negative symptom spectra.
Conflict of interest statement
Figures





Similar articles
-
Altered CSMD1 Expression Alters Cocaine-Conditioned Place Preference: Mutual Support for a Complex Locus from Human and Mouse Models.PLoS One. 2015 Jul 14;10(7):e0120908. doi: 10.1371/journal.pone.0120908. eCollection 2015. PLoS One. 2015. PMID: 26171607 Free PMC article.
-
Assessment of behaviors modeling aspects of schizophrenia in Csmd1 mutant mice.PLoS One. 2012;7(12):e51235. doi: 10.1371/journal.pone.0051235. Epub 2012 Dec 17. PLoS One. 2012. PMID: 23284669 Free PMC article.
-
The schizophrenia-associated gene CSMD1 encodes a complement classical pathway inhibitor predominantly expressed by astrocytes and at synapses in mice and humans.Brain Behav Immun. 2025 Jul;127:287-302. doi: 10.1016/j.bbi.2025.03.026. Epub 2025 Mar 18. Brain Behav Immun. 2025. PMID: 40112933
-
The Diverse Role of CUB and Sushi Multiple Domains 1 (CSMD1) in Human Diseases.Genes (Basel). 2022 Dec 10;13(12):2332. doi: 10.3390/genes13122332. Genes (Basel). 2022. PMID: 36553598 Free PMC article. Review.
-
The complement system in schizophrenia: where are we now and what's next?Mol Psychiatry. 2020 Jan;25(1):114-130. doi: 10.1038/s41380-019-0479-0. Epub 2019 Aug 22. Mol Psychiatry. 2020. PMID: 31439935 Review.
Cited by
-
Complement Activation in the Central Nervous System: A Biophysical Model for Immune Dysregulation in the Disease State.Front Mol Neurosci. 2021 Mar 4;14:620090. doi: 10.3389/fnmol.2021.620090. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33746710 Free PMC article.
-
Genome-wide association study identifying novel variant for fasting insulin and allelic heterogeneity in known glycemic loci in Chilean adolescents: The Santiago Longitudinal Study.Pediatr Obes. 2021 Jul;16(7):e12765. doi: 10.1111/ijpo.12765. Epub 2020 Dec 30. Pediatr Obes. 2021. PMID: 33381925 Free PMC article.
-
Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function.Front Immunol. 2018 Apr 13;9:698. doi: 10.3389/fimmu.2018.00698. eCollection 2018. Front Immunol. 2018. PMID: 29706957 Free PMC article. Review.
-
Gene-environment interactions in the influence of maternal education on adolescent neurodevelopment using ABCD study.Sci Adv. 2024 Nov 15;10(46):eadp3751. doi: 10.1126/sciadv.adp3751. Epub 2024 Nov 15. Sci Adv. 2024. PMID: 39546599 Free PMC article.
-
Altered CSMD1 Expression Alters Cocaine-Conditioned Place Preference: Mutual Support for a Complex Locus from Human and Mouse Models.PLoS One. 2015 Jul 14;10(7):e0120908. doi: 10.1371/journal.pone.0120908. eCollection 2015. PLoS One. 2015. PMID: 26171607 Free PMC article.
References
-
- Dernovšek MZ, Šprah L (2009) Comorbid anxiety in patients with psychosis. Psychiatr Danub 21 Suppl 143–50. - PubMed
-
- Håvik B, Røkke H, Dagyte G, Stavrum AK, Bramham CR, et al. (2007) Synaptic activity-induced global gene expression patterns in the dentate gyrus of adult behaving rats: induction of immunity-linked genes. Neuroscience 148: 925–936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

