Hearing loss in a mouse model of 22q11.2 Deletion Syndrome
- PMID: 24244619
- PMCID: PMC3828191
- DOI: 10.1371/journal.pone.0080104
Hearing loss in a mouse model of 22q11.2 Deletion Syndrome
Abstract
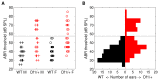
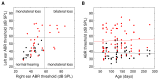
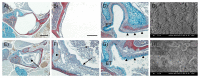
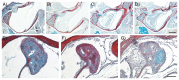
22q11.2 Deletion Syndrome (22q11DS) arises from an interstitial chromosomal microdeletion encompassing at least 30 genes. This disorder is one of the most significant known cytogenetic risk factors for schizophrenia, and can also cause heart abnormalities, cognitive deficits, hearing difficulties, and a variety of other medical problems. The Df1/+ hemizygous knockout mouse, a model for human 22q11DS, recapitulates many of the deficits observed in the human syndrome including heart defects, impaired memory, and abnormal auditory sensorimotor gating. Here we show that Df1/+ mice, like human 22q11DS patients, have substantial rates of hearing loss arising from chronic middle ear infection. Auditory brainstem response (ABR) measurements revealed significant elevation of click-response thresholds in 48% of Df1/+ mice, often in only one ear. Anatomical and histological analysis of the middle ear demonstrated no gross structural abnormalities, but frequent signs of otitis media (OM, chronic inflammation of the middle ear), including excessive effusion and thickened mucosa. In mice for which both in vivo ABR thresholds and post mortem middle-ear histology were obtained, the severity of signs of OM correlated directly with the level of hearing impairment. These results suggest that abnormal auditory sensorimotor gating previously reported in mouse models of 22q11DS could arise from abnormalities in auditory processing. Furthermore, the findings indicate that Df1/+ mice are an excellent model for increased risk of OM in human 22q11DS patients. Given the frequently monaural nature of OM in Df1/+ mice, these animals could also be a powerful tool for investigating the interplay between genetic and environmental causes of OM.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

