Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm
- PMID: 24244642
- PMCID: PMC3828235
- DOI: 10.1371/journal.pone.0080181
Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm
Abstract
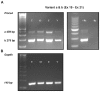
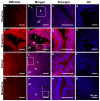
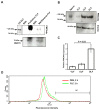
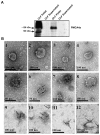
PMCA4, a membrane protein, is the major Ca(2+) efflux pump in murine sperm where its deletion leads to a severe loss of hyperactivated motility and to male infertility. We have previously shown that the PMCA4b splice variant interacts with CASK (Ca(2+/)CaM-dependent serine kinase) in regulating sperm Ca(2+). More recently we detected that PMCA4a isoform, in addition to its presence in testis, is secreted in the epididymal luminal fluid and transferred to sperm. Here we show that Pmca4 mRNA is expressed in both the 4a and 4b variants in the vagina, uterus, and oviduct. Immunofluorescence reveals that PMCA4a is similarly expressed and is elevated during estrus, appearing in the glandular and luminal epithelia. Western analysis detected PMCA4a in all tissues and in the luminal fluids (LF) of the vagina (VLF), uterus (ULF), and the oviduct (OLF) collected during estrus. It was ~9- and 4-fold higher in OLF than in VLF and ULF, and only marginally present in LF collected at metestrus/diestrus. Fractionation of the LF collected at estrus, via ultracentrifugation, revealed that 100% of the PMCA4a resides in the vesicular fraction of the ULF and OLF. Transmission electron microscopy (TEM) revealed that OLF vesicles have an exosomal orientation (with the cytoplasmic-side inward), a size range of 25-100 nm, with the characteristic CD9 biomarker. Thus, we dubbed these vesicles "oviductosomes", to which PMCA4a was immunolocalized. Incubation of caudal sperm in the combined LF or exosomes resulted in up to a ~3-fold increase of sperm PMCA4a, as detected by flow cytometry, indicating in vitro uptake. Our results are consistent with the increased requirement of Ca(2+) efflux in the oviduct. They show for the first time the presence of oviductal exosomes and highlight their role, along with uterosomes and vaginal exosomes, in post-testicular sperm acquisition of PMCA4a which is essential for hyperactivated motility and fertility.
Conflict of interest statement
Figures




References
-
- Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT et al. (2004) Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279: 33742-33750. doi:10.1074/jbc.M404628200. PubMed: 15178683. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

