Annexin A4 is involved in proliferation, chemo-resistance and migration and invasion in ovarian clear cell adenocarcinoma cells
- PMID: 24244679
- PMCID: PMC3823662
- DOI: 10.1371/journal.pone.0080359
Annexin A4 is involved in proliferation, chemo-resistance and migration and invasion in ovarian clear cell adenocarcinoma cells
Abstract
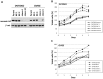
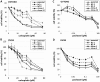
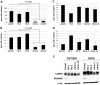
Ovarian clear cell adenocarcinoma (CCC) is the second most common subtype of ovarian cancer after high-grade serous adenocarcinomas. CCC tends to develop resistance to the standard platinum-based chemotherapy, and has a poor prognosis when diagnosed in advanced stages. The ANXA4 gene, along with its product, a Ca(++)-binding annexin A4 (ANXA4) protein, has been identified as the CCC signature gene. We reported two subtypes of ANXA4 with different isoelectric points (IEPs) that are upregulated in CCC cell lines. Although several in vitro investigations have shown ANXA4 to be involved in cancer cell proliferation, chemoresistance, and migration, these studies were generally based on its overexpression in cells other than CCC. To elucidate the function of the ANXA4 in CCC cells, we established CCC cell lines whose ANXA4 expressions are stably knocked down. Two parental cells were used: OVTOKO contains almost exclusively an acidic subtype of ANXA4, and OVISE contains predominantly a basic subtype but also a detectable acidic subtype. ANXA4 knockdown (KO) resulted in significant growth retardation and greater sensitivity to carboplatin in OVTOKO cells. ANXA4-KO caused significant loss of migration and invasion capability in OVISE cells, but this effect was not seen in OVTOKO cells. We failed to find the cause of the different IEPs of ANXA4, but confirmed that the two subtypes are found in clinical CCC samples in ratios that vary by patient. Further investigation to clarify the mechanism that produces the subtypes is needed to clarify the function of ANXA4 in CCC, and might allow stratification and improved treatment strategies for patients with CCC.
Conflict of interest statement
Figures



References
-
- Gurung A, Hung T, Morin J, Gilks CB (2013) Molecular abnormalities in ovarian carcinoma: clinical, morphological and therapeutic correlates. Histopathology 62: 59–70. - PubMed
-
- Anglesio MS, Carey MS, Kobel M, Mackay H, Huntsman DG (2011) Clear cell carcinoma of the ovary: a report from the first Ovarian Clear Cell Symposium, June 24th, 2010. Gynecol Oncol 121: 407–415. - PubMed
-
- Schwartz DR, Kardia SL, Shedden KA, Kuick R, Michailidis G, et al. (2002) Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res 62: 4722–4729. - PubMed
-
- Tsuchiya A, Sakamoto M, Yasuda J, Chuma M, Ohta T, et al. (2003) Expression profiling in ovarian clear cell carcinoma: identification of hepatocyte nuclear factor-1 beta as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am J Pathol 163: 2503–2512. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

