Role of glucuronidation for hepatic detoxification and urinary elimination of toxic bile acids during biliary obstruction
- PMID: 24244729
- PMCID: PMC3828276
- DOI: 10.1371/journal.pone.0080994
Role of glucuronidation for hepatic detoxification and urinary elimination of toxic bile acids during biliary obstruction
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/ef13ed51-6848-419d-94d8-1bb62e7bcf52
Abstract
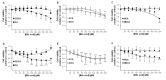
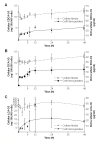
Biliary obstruction, a severe cholestatic condition, results in a huge accumulation of toxic bile acids (BA) in the liver. Glucuronidation, a conjugation reaction, is thought to protect the liver by both reducing hepatic BA toxicity and increasing their urinary elimination. The present study evaluates the contribution of each process in the overall BA detoxification by glucuronidation. Glucuronide (G), glycine, taurine conjugates, and unconjugated BAs were quantified in pre- and post-biliary stenting urine samples from 12 patients with biliary obstruction, using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The same LC-MS/MS procedure was used to quantify intra- and extracellular BA-G in Hepatoma HepG2 cells. Bile acid-induced toxicity in HepG2 cells was evaluated using MTS reduction, caspase-3 and flow cytometry assays. When compared to post-treatment samples, pre-stenting urines were enriched in glucuronide-, taurine- and glycine-conjugated BAs. Biliary stenting increased the relative BA-G abundance in the urinary BA pool, and reduced the proportion of taurine- and glycine-conjugates. Lithocholic, deoxycholic and chenodeoxycholic acids were the most cytotoxic and pro-apoptotic/necrotic BAs for HepG2 cells. Other species, such as the cholic, hyocholic and hyodeoxycholic acids were nontoxic. All BA-G assayed were less toxic and displayed lower pro-apoptotic/necrotic effects than their unconjugated precursors, even if they were able to penetrate into HepG2 cells. Under severe cholestatic conditions, urinary excretion favors the elimination of amidated BAs, while glucuronidation allows the conversion of cytotoxic BAs into nontoxic derivatives.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

