Nup358 interacts with Dishevelled and aPKC to regulate neuronal polarity
- PMID: 24244865
- PMCID: PMC3828775
- DOI: 10.1242/bio.20135363
Nup358 interacts with Dishevelled and aPKC to regulate neuronal polarity
Abstract
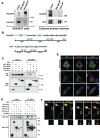
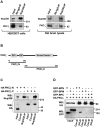
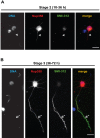
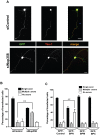
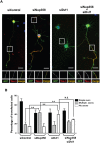
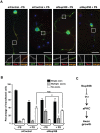
Par polarity complex, consisting of Par3, Par6, and aPKC, plays a conserved role in the establishment and maintenance of polarization in diverse cellular contexts. Recent reports suggest that Dishevelled (Dvl), a cytoplasmic mediator of Wnt signalling, interacts with atypical protein kinase C and regulates its activity during neuronal differentiation and directed cell migration. Here we show that Nup358 (also called RanBP2), a nucleoporin previously implicated in polarity during directed cell migration, interacts with Dishevelled and aPKC through its N-terminal region (BPN) and regulates axon-dendrite differentiation of cultured hippocampal neurons. Depletion of endogenous Nup358 leads to generation of multiple axons, whereas overexpression of BPN abrogates the process of axon formation. Moreover, siRNA-mediated knockdown of Dvl or inhibition of aPKC by a pseudosubstrate inhibitor significantly reverses the multiple axon phenotype produced by Nup358 depletion. Collectively, these data suggest that Nup358 plays an important role in regulating neuronal polarization upstream to Dvl and aPKC.
Keywords: Dishevelled; Neuron; Nucleoporin; Nup358; Polarity; aPKC.
Conflict of interest statement
Figures
Similar articles
-
PAR3-PAR6-atypical PKC polarity complex proteins in neuronal polarization.Cell Mol Life Sci. 2018 Aug;75(15):2735-2761. doi: 10.1007/s00018-018-2828-6. Epub 2018 Apr 25. Cell Mol Life Sci. 2018. PMID: 29696344 Free PMC article. Review.
-
Dishevelled promotes axon differentiation by regulating atypical protein kinase C.Nat Cell Biol. 2007 Jul;9(7):743-54. doi: 10.1038/ncb1603. Epub 2007 Jun 10. Nat Cell Biol. 2007. PMID: 17558396
-
Nup358 regulates microridge length by controlling SUMOylation-dependent activity of aPKC in zebrafish epidermis.J Cell Sci. 2019 Jun 17;132(12):jcs224501. doi: 10.1242/jcs.224501. J Cell Sci. 2019. PMID: 31164446
-
Regulation of aPKC activity by Nup358 dependent SUMO modification.Sci Rep. 2016 Sep 29;6:34100. doi: 10.1038/srep34100. Sci Rep. 2016. PMID: 27682244 Free PMC article.
-
The Roles of Par3, Par6, and aPKC Polarity Proteins in Normal Neurodevelopment and in Neurodegenerative and Neuropsychiatric Disorders.J Neurosci. 2022 Jun 15;42(24):4774-4793. doi: 10.1523/JNEUROSCI.0059-22.2022. J Neurosci. 2022. PMID: 35705493 Free PMC article. Review.
Cited by
-
Varroa destructor parasitism has a greater effect on proteome changes than the deformed wing virus and activates TGF-β signaling pathways.Sci Rep. 2019 Jun 28;9(1):9400. doi: 10.1038/s41598-019-45764-1. Sci Rep. 2019. PMID: 31253851 Free PMC article.
-
Nuclear envelope and chromatin choreography direct cellular differentiation.Nucleus. 2025 Dec;16(1):2449520. doi: 10.1080/19491034.2024.2449520. Epub 2025 Feb 12. Nucleus. 2025. PMID: 39943681 Free PMC article. Review.
-
PAR3-PAR6-atypical PKC polarity complex proteins in neuronal polarization.Cell Mol Life Sci. 2018 Aug;75(15):2735-2761. doi: 10.1007/s00018-018-2828-6. Epub 2018 Apr 25. Cell Mol Life Sci. 2018. PMID: 29696344 Free PMC article. Review.
-
TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD.Nat Neurosci. 2018 Feb;21(2):228-239. doi: 10.1038/s41593-017-0047-3. Epub 2018 Jan 8. Nat Neurosci. 2018. PMID: 29311743 Free PMC article.
-
Nup358 binds to AGO proteins through its SUMO-interacting motifs and promotes the association of target mRNA with miRISC.EMBO Rep. 2017 Feb;18(2):241-263. doi: 10.15252/embr.201642386. Epub 2016 Dec 30. EMBO Rep. 2017. PMID: 28039207 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

