Building a multifunctional aptamer-based DNA nanoassembly for targeted cancer therapy
- PMID: 24245521
- PMCID: PMC3891927
- DOI: 10.1021/ja4094617
Building a multifunctional aptamer-based DNA nanoassembly for targeted cancer therapy
Abstract
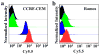
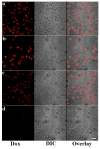
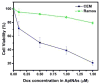
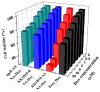
The ability to self-assemble one-dimensional DNA building blocks into two- and three-dimensional nanostructures via DNA/RNA nanotechnology has led to broad applications in bioimaging, basic biological mechanism studies, disease diagnosis, and drug delivery. However, the cellular uptake of most nucleic acid nanostructures is dependent on passive delivery or the enhanced permeability and retention effect, which may not be suitable for certain types of cancers, especially for treatment in vivo. To meet this need, we have constructed a multifunctional aptamer-based DNA nanoassembly (AptNA) for targeted cancer therapy. In particular, we first designed various Y-shaped functional DNA domains through predesigned base pair hybridization, including targeting aptamers, intercalated anticancer drugs, and therapeutic antisense oligonucleotides. Then these functional DNA domains were linked to an X-shaped DNA core connector, termed a building unit, through the complementary sequences in the arms of functional domains and connector. Finally, hundreds (~100-200) of these basic building units with 5'-modification of acrydite groups were further photo-cross-linked into a multifunctional and programmable aptamer-based nanoassembly structure able to take advantage of facile modular design and assembly, high programmability, excellent biostability and biocompatibility, as well as selective recognition and transportation. With these properties, AptNAs were demonstrated to have specific cytotoxic effect against leukemia cells. Moreover, the incorporation of therapeutic antisense oligonucleotides resulted in the inhibition of P-gp expression (a drug efflux pump to increase excretion of anticancer drugs) as well as a decrease in drug resistance. Therefore, these multifunctional and programmable aptamer-based DNA nanoassemblies show promise as candidates for targeted drug delivery and cancer therapy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nano. 2007;2:751. - PubMed
-
- Petros RA, DeSimone JM. Nat Rev Drug Discov. 2010;9:615. - PubMed
-
- Li Y, Tseng YD, Kwon SY, d’Espaux L, Bunch JS, McEuen PL, Luo D. Nat Mater. 2004;3:38. - PubMed
-
- Goodman RP, Schaap IAT, Tardin CF, Erben CM, Berry RM, Schmidt CF, Turberfield AJ. Science. 2005;310:1661. - PubMed
-
- Aldaye FA, Palmer AL, Sleiman HF. Science. 2008;321:1795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

