New approaches to target the mycolic acid biosynthesis pathway for the development of tuberculosis therapeutics
- PMID: 24245756
- PMCID: PMC4568743
- DOI: 10.2174/1381612819666131118203641
New approaches to target the mycolic acid biosynthesis pathway for the development of tuberculosis therapeutics
Abstract
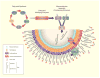
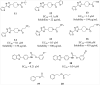
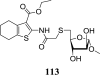
Mycolic acids are the major lipid components of the unique mycobacterial cell wall responsible for the protection of the tuberculosis bacilli from many outside threats. Mycolic acids are synthesized in the cytoplasm and transported to the outer membrane as trehalose- containing glycolipids before being esterified to the arabinogalactan portion of the cell wall and outer membrane glycolipids. The large size of these unique fatty acids is a result of a huge metabolic investment that has been evolutionarily conserved, indicating the importance of these lipids to the mycobacterial cellular survival. There are many key enzymes involved in the mycolic acid biosynthetic pathway, including fatty acid synthesis (KasA, KasB, MabA, InhA, HadABC), mycolic acid modifying enzymes (SAM-dependent methyltransferases, aNAT), fatty acid activating and condensing enzymes (FadD32, Acc, Pks13), transporters (MmpL3) and tranferases (Antigen 85A-C) all of which are excellent potential drug targets. Not surprisingly, in recent years many new compounds have been reported to inhibit specific portions of this pathway, discovered through both phenotypic screening and target enzyme screening. In this review, we analyze the new and emerging inhibitors of this pathway discovered in the post-genomic era of tuberculosis drug discovery, several of which show great promise as selective tuberculosis therapeutics.
Figures

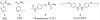




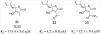



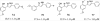









References
-
- Global tuberculosis report 2012. World Health Organization; 2012.
-
- WHO. Treatment of tuberculosis: guidelines for national programmes. 4th. Geneva, Switzerland: World Health Organization; 2010.
-
- Jain A, Mondal R. Extensively drug-resistant tuberculosis: current challenges and threats. FEMS Immunology & Medicinal Microbiology. 2008;53:145–50. - PubMed
-
- Raviglione MD, Snider DE, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwid epidemic. JAMA : the journal of the American Medical Association. 1995;273(3):220–6. - PubMed
-
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis. Estimated incidence, prevalence and mortality by country. JAMA : the journal of the American Medical Association. 1999;282(7):677–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

