Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition of cell proliferation and triggers apoptosis in cervical cancer cells
- PMID: 24245877
- PMCID: PMC4176749
- DOI: 10.1186/2047-783X-18-46
Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition of cell proliferation and triggers apoptosis in cervical cancer cells
Abstract
Background: The aim of this study is to investigate the effects of polyphenol extract from Phyllanthus emblica (PEEP) on cervical cancer cells and to explore the underlying mechanism.
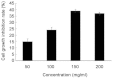
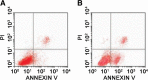
Methods: MTT assay was used to measure inhibition of proliferation of cervical cancer (HeLa) cells after treatment with PEEP at concentrations of 0, 50, 100, 150, and 200 mg/ml for 48 hours. HeLa cells were treated with PEEP (150 mg/ml) for 48 hours in the following analysis. Karyomorphism was assessed by immunofluorescence using DAPI staining, and cell apoptosis and cell cycle were assessed using flow cytometry. Three apoptotic marker proteins, namely, Fas, FasL, and cleaved caspase-8, were assessed by western blotting.
Results: PEEP inhibited the growth of HeLa cells, and the optimum concentration of PEEP was 150 mg/ml. In addition, the karyomorphism of HeLa cells after treatment with PEEP was abnormal. Furthermore, PEEP induced arrest of the HeLa cell cycle at G2/M phase, and triggered apoptosis. PEEP also induced significant Fas and FasL activation, and cleavage of caspase-8.
Conclusions: Our study indicates that PEEP is effective in inhibiting HeLa cell proliferation by inducing cell cycle arrest at G2/M phase and inducing apoptosis.
Figures



References
-
- Kleinerman RA, Boice JD Jr, Storm HH, Sparen P, Andersen A, Pukkala E, Lynch CF, Hankey BF, Flannery JT. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer. 1995;76:442–452. doi: 10.1002/1097-0142(19950801)76:3<442::AID-CNCR2820760315>3.0.CO;2-L. - DOI - PubMed
-
- Yumin W, Jie C, Wenhui Z, Wangdong H, Fangyou Y. Study of the prevalence of human Papillomavirus infection in Chinese women with cervical cancer. Afr J Microbiol Res. 2012;6:1048–1053.
-
- Kumar V, Robbins SL. Robbins Basic Pathology. 8. Philadelphia, PA: Saunders/Elsevier; 2007.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

