Plasmodium falciparum picks (on) EPCR
- PMID: 24246501
- PMCID: PMC3888284
- DOI: 10.1182/blood-2013-09-521005
Plasmodium falciparum picks (on) EPCR
Abstract
Of all the outcomes of Plasmodium falciparum infection, the coma of cerebral malaria (CM) is particularly deadly. Malariologists have long wondered how some patients develop this organ-specific syndrome. Data from two recent publications support a novel mechanism of CM pathogenesis in which infected erythrocytes (IEs) express specific virulence proteins that mediate IE binding to the endothelial protein C receptor (EPCR). Malaria-associated depletion of EPCR, with subsequent impairment of the protein C system promotes a proinflammatory, procoagulant state in brain microvessels.
Figures
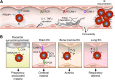

Comment in
-
EPCR: holy grail of malaria cytoadhesion?Blood. 2014 Jan 9;123(2):157-9. doi: 10.1182/blood-2013-12-541318. Blood. 2014. PMID: 24408207
References
-
- Weatherall DJ, Miller LH, Baruch DI, et al. Malaria and the red cell. Hematology Am Soc Hematol Educ Program. 2002:35–57. - PubMed
-
- Arap W, Kolonin MG, Trepel M, et al. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8(2):121–127. - PubMed
-
- Knowles DM, II, Tolidjian B, Marboe C, D’Agati V, Grimes M, Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984;132(5):2170–2173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

