Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis
- PMID: 24246855
- PMCID: PMC5802971
- DOI: 10.1016/j.yfrne.2013.11.001
Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis
Abstract
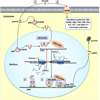
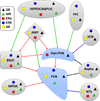
The hypothalamo-pituitary-adrenal (HPA) axis represents a complex neuroendocrine feedback loop controlling the secretion of adrenal glucocorticoid hormones. Central to its function is the paraventricular nucleus of the hypothalamus (PVN) where neurons expressing corticotropin releasing factor reside. These HPA motor neurons are a primary site of integration leading to graded endocrine responses to physical and psychological stressors. An important regulatory factor that must be considered, prior to generating an appropriate response is the animal's reproductive status. Thus, PVN neurons express androgen and estrogen receptors and receive input from sites that also express these receptors. Consequently, changes in reproduction and gonadal steroid levels modulate the stress response and this underlies sex differences in HPA axis function. This review examines the make up of the HPA axis and hypothalamo-pituitary-gonadal (HPG) axis and the interactions between the two that should be considered when exploring normal and pathological responses to environmental stressors.
Keywords: Androgen receptor; CRF; Dihydrotestosterone; Estradiol; Estrogen receptor; Glucocorticoid; HPA; HPG; Sex difference; Testosterone.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Abel K, Majzoub JA. Molecular biology of the HPA axis. In: Steckler T, Kalin NH, Reul JM, editors. Handbook of Stress and the Brain. Vol. 15. Elsevier; 2005. pp. 79–94.
-
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. - PubMed
-
- Adan RA, Cox JJ, Beischlag TV, Burbach JP. A composite hormone response element mediates the transactivation of the rat oxytocin gene by different classes of nuclear hormone receptors. Mol Endocrinol. 1993;7:47–57. - PubMed
-
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. - PubMed
-
- Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides. 2004;25:319–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

