Traumatic injury to the immature frontal lobe: a new murine model of long-term motor impairment in the absence of psychosocial or cognitive deficits
- PMID: 24247103
- PMCID: PMC3923401
- DOI: 10.1159/000355874
Traumatic injury to the immature frontal lobe: a new murine model of long-term motor impairment in the absence of psychosocial or cognitive deficits
Abstract
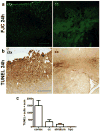
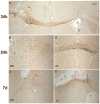
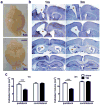
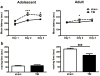
Traumatic brain injury in children commonly involves the frontal lobes and is associated with distinct structural and behavioral changes. Despite the clinical significance of injuries localized to this region during brain development, the mechanisms underlying secondary damage and long-term recovery are poorly understood. Here, we have characterized the first model of unilateral focal traumatic injury to the developing frontal lobe. Male C57Bl/6J mice at postnatal day (p)21, an age approximating a toddler-aged child, received a controlled cortical impact or sham surgery to the left frontal lobe and were euthanized 1 or 7 days later. A necrotic cavity and local inflammatory response were largely confined to the unilateral frontal lobe, dorsal corpus callosum and striatum anterior to the bregma. While cell death and accumulated β-amyloid precursor protein were characteristic features of the pericontusional motor cortex, corpus callosum, cingulum and dorsal striatum, underlying structures including the hippocampus showed no overt pathology. To determine the long-term functional consequences of injury at p21, two additional cohorts were subjected to a battery of behavioral tests in adolescence (p35-45) or adulthood (p70-80). In both cohorts, brain-injured mice showed normal levels of anxiety, sociability, spatial learning and memory. The signature phenotypic features were deficits in motor function and motor learning, coincident with a reduction in ipsilateral cortical brain volumes. Together, these findings demonstrate classic morphological features of a focal traumatic injury, including early cell death and axonal injury, and long-term volumetric loss of cortical volumes. The presence of deficits in sensorimotor function and coordination in the absence of abnormal findings related to anxiety, sociability and memory likely reflects several variables, including the unique location of the injury and the emergence of favorable compensatory mechanisms during subsequent brain development.
© 2013 S. Karger AG, Basel.
Figures



Similar articles
-
Tau reduction diminishes spatial learning and memory deficits after mild repetitive traumatic brain injury in mice.PLoS One. 2014 Dec 31;9(12):e115765. doi: 10.1371/journal.pone.0115765. eCollection 2014. PLoS One. 2014. PMID: 25551452 Free PMC article.
-
Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function.J Neurotrauma. 2011 Apr;28(4):547-63. doi: 10.1089/neu.2010.1729. J Neurotrauma. 2011. PMID: 21299360 Free PMC article.
-
Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse.J Neurotrauma. 1998 Aug;15(8):599-614. doi: 10.1089/neu.1998.15.599. J Neurotrauma. 1998. PMID: 9726259
-
Neurobiology of adolescent substance use disorders: implications for prevention and treatment.Child Adolesc Psychiatr Clin N Am. 2010 Jul;19(3):479-92. doi: 10.1016/j.chc.2010.03.003. Child Adolesc Psychiatr Clin N Am. 2010. PMID: 20682216 Free PMC article. Review.
-
Factors influencing frontal cortex development and recovery from early frontal injury.Dev Neurorehabil. 2009;12(5):269-78. doi: 10.3109/17518420903087715. Dev Neurorehabil. 2009. PMID: 20477557 Free PMC article. Review.
Cited by
-
Early Gelatinase Activity Is Not a Determinant of Long-Term Recovery after Traumatic Brain Injury in the Immature Mouse.PLoS One. 2015 Nov 20;10(11):e0143386. doi: 10.1371/journal.pone.0143386. eCollection 2015. PLoS One. 2015. PMID: 26588471 Free PMC article.
-
Chronic mTORC1 inhibition rescues behavioral and biochemical deficits resulting from neuronal Depdc5 loss in mice.Hum Mol Genet. 2019 Sep 1;28(17):2952-2964. doi: 10.1093/hmg/ddz123. Hum Mol Genet. 2019. PMID: 31174205 Free PMC article.
-
A novel antagonist of p75NTR reduces peripheral expansion and CNS trafficking of pro-inflammatory monocytes and spares function after traumatic brain injury.J Neuroinflammation. 2016 Apr 22;13(1):88. doi: 10.1186/s12974-016-0544-4. J Neuroinflammation. 2016. PMID: 27102880 Free PMC article.
-
Effects of PM2.5 and gases exposure during prenatal and early-life on autism-like phenotypes in male rat offspring.Part Fibre Toxicol. 2020 Jan 29;17(1):8. doi: 10.1186/s12989-020-0336-y. Part Fibre Toxicol. 2020. PMID: 31996222 Free PMC article.
-
A New Rabbit Model of Pediatric Traumatic Brain Injury.J Neurotrauma. 2015 Sep 1;32(17):1369-79. doi: 10.1089/neu.2014.3701. Epub 2015 May 15. J Neurotrauma. 2015. PMID: 25758339 Free PMC article.
References
-
- Langlois JA. Traumatic Brain Injury in the United States: Assessing outcomes in children. Atlanta, GA: National Center for Injury Prevention and Control; 2000. http://www.cdc.gov/traumaticbraininjury/pdf/TBI_assessing.pdf.
-
- Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. 10 years outcome from childhood traumatic brain injury. Int J Dev Neurosci. 2012;30:217–224. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

