Sepsis-induced potentiation of peritoneal macrophage migration is mitigated by programmed cell death receptor-1 gene deficiency
- PMID: 24247196
- PMCID: PMC3972305
- DOI: 10.1159/000355888
Sepsis-induced potentiation of peritoneal macrophage migration is mitigated by programmed cell death receptor-1 gene deficiency
Abstract
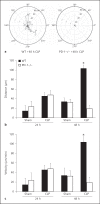
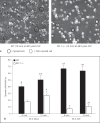
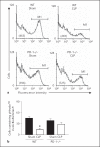
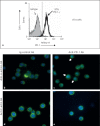
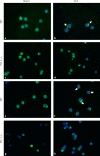
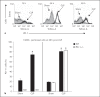
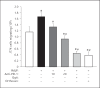
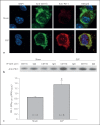
The effect of programmed cell death receptor-1 (PD-1) on phagocyte function has not been extensively described. Here we report that experimental mouse sepsis, cecal ligation and puncture (CLP), induced a marked increase in peritoneal macrophage random migration, motility and cell spread, but these changes were lost in the absence of PD-1. Alternatively, phagocytic activity was inversely affected. In vitro cell culture imaging studies, with the macrophage cell line J774, documented that blocking PD-1 with antibody led to aggregation of the cytoskeletal proteins α-actinin and F-actin. Further experiments looking at ex vivo peritoneal macrophages from mice illustrated that a similar pattern of α-actinin and F-actin was evident on cells from wild-type CLP mice but not PD-1-/- CLP mouse cells. We also observed that fMLP-induced migration by J774 cells was markedly attenuated using PD-1 blocking antibodies, a nonselective phosphatase inhibitor and a selective Ras-related protein 1 inhibitor. Finally, peritoneal macrophages derived from CLP as opposed to Sham mice demonstrated aspects of both cell surface co-localization with CD11b and internalization of PD-1 within vacuoles independent of CD11b staining. Together, we believe the data support a role for PD-1 in mediating aspects of innate macrophage immune dysfunction during sepsis, heretofore unappreciated.
© 2013 S. Karger AG, Basel.
Figures

Similar articles
-
A novel role for programmed cell death receptor ligand 2 in sepsis-induced hepatic dysfunction.Am J Physiol Gastrointest Liver Physiol. 2019 Jan 1;316(1):G106-G114. doi: 10.1152/ajpgi.00204.2018. Epub 2018 Nov 15. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30431333 Free PMC article.
-
Contribution of programmed cell death receptor (PD)-1 to Kupffer cell dysfunction in murine polymicrobial sepsis.Am J Physiol Gastrointest Liver Physiol. 2016 Aug 1;311(2):G237-45. doi: 10.1152/ajpgi.00371.2015. Epub 2016 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27288425 Free PMC article.
-
Identification of B7-H1 as a novel mediator of the innate immune/proinflammatory response as well as a possible myeloid cell prognostic biomarker in sepsis.J Immunol. 2014 Feb 1;192(3):1091-9. doi: 10.4049/jimmunol.1302252. Epub 2013 Dec 30. J Immunol. 2014. PMID: 24379123 Free PMC article.
-
BMAL2 promotes eCIRP-induced macrophage endotoxin tolerance.Front Immunol. 2024 Jun 13;15:1426682. doi: 10.3389/fimmu.2024.1426682. eCollection 2024. Front Immunol. 2024. PMID: 38938563 Free PMC article.
-
PD-1 signaling pathway in sepsis: Does it have a future?Clin Immunol. 2021 Aug;229:108742. doi: 10.1016/j.clim.2021.108742. Epub 2021 Apr 24. Clin Immunol. 2021. PMID: 33905818 Review.
Cited by
-
The pathogenesis and potential therapeutic targets in sepsis.MedComm (2020). 2023 Nov 20;4(6):e418. doi: 10.1002/mco2.418. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38020710 Free PMC article. Review.
-
The dysfunction of complement and coagulation in diseases: the implications for the therapeutic interventions.MedComm (2020). 2024 Oct 23;5(11):e785. doi: 10.1002/mco2.785. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39445002 Free PMC article. Review.
-
4-Octyl itaconate regulates immune balance by activating Nrf2 and negatively regulating PD-L1 in a mouse model of sepsis.Int J Biol Sci. 2022 Oct 24;18(16):6189-6209. doi: 10.7150/ijbs.74456. eCollection 2022. Int J Biol Sci. 2022. PMID: 36439878 Free PMC article.
-
Application of Lung-Targeted Lipid Nanoparticle-delivered mRNA of soluble PD-L1 via SORT Technology in Acute Respiratory Distress Syndrome.Theranostics. 2023 Sep 4;13(14):4974-4992. doi: 10.7150/thno.86466. eCollection 2023. Theranostics. 2023. PMID: 37771784 Free PMC article.
-
The implication of targeting PD-1:PD-L1 pathway in treating sepsis through immunostimulatory and anti-inflammatory pathways.Front Immunol. 2023 Dec 13;14:1323797. doi: 10.3389/fimmu.2023.1323797. eCollection 2023. Front Immunol. 2023. PMID: 38193090 Free PMC article. Review.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58:867–874. - PubMed
-
- Rice TW, Bernard GR. Therapeutic interventions and targets for sepsis. Annu Rev Med. 2005;56:225–248. - PubMed
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. - PubMed
-
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2001;31:1250–1256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

