Molecular basis of ADP inhibition of vacuolar (V)-type ATPase/synthase
- PMID: 24247239
- PMCID: PMC3879562
- DOI: 10.1074/jbc.M113.523498
Molecular basis of ADP inhibition of vacuolar (V)-type ATPase/synthase
Abstract
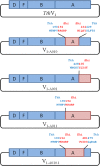
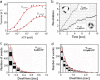
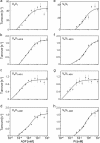
Reduction of ATP hydrolysis activity of vacuolar-type ATPase/synthase (V0V1) as a result of ADP inhibition occurs as part of the normal mechanism of V0V1 of Thermus thermophilus but not V0V1 of Enterococcus hirae or eukaryotes. To investigate the molecular basis for this difference, domain-swapped chimeric V1 consisting of both T. thermophilus and E. hirae enzymes were generated, and their function was analyzed. The data showed that the interaction between the nucleotide binding and C-terminal domains of the catalytic A subunit from E. hirae V1 is central to increasing binding affinity of the chimeric V1 for phosphate, resulting in reduction of the ADP inhibition. These findings together with a comparison of the crystal structures of T. thermophilus V1 with E. hirae V1 strongly suggest that the A subunit adopts a conformation in T. thermophilus V1 different from that in E. hirae V1. This key difference results in ADP inhibition of T. thermophilus V1 by abolishing the binding affinity for phosphate during ATP hydrolysis.
Keywords: ADP Inhibition; ATP Synthase; F1F0 ATPase; Membrane Proteins; Molecular Motors; Vacuolar ATPase.
Figures







References
-
- Forgac M. (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929 - PubMed
-
- Muench S. P., Trinick J., Harrison M. A. (2011) Structural divergence of the rotary ATPases. Q. Rev. Biophys. 44, 311–356 - PubMed
-
- Yokoyama K., Imamura H. (2005) Rotation, structure, and classification of prokaryotic V-ATPase. J. Bioenerg. Biomembr. 37, 405–410 - PubMed
-
- Murata T., Igarashi K., Kakinuma Y., Yamato I. (2000) Na+ binding of V-type Na+-ATPase in Enterococcus hirae. J. Biol. Chem. 275, 13415–13419 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

