Mammalian protein arginine methyltransferase 7 (PRMT7) specifically targets RXR sites in lysine- and arginine-rich regions
- PMID: 24247247
- PMCID: PMC3873558
- DOI: 10.1074/jbc.M113.525345
Mammalian protein arginine methyltransferase 7 (PRMT7) specifically targets RXR sites in lysine- and arginine-rich regions
Abstract
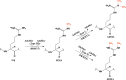
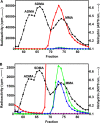
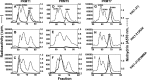
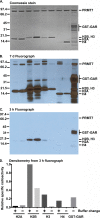
The mammalian protein arginine methyltransferase 7 (PRMT7) has been implicated in roles of transcriptional regulation, DNA damage repair, RNA splicing, cell differentiation, and metastasis. However, the type of reaction that it catalyzes and its substrate specificity remain controversial. In this study, we purified a recombinant mouse PRMT7 expressed in insect cells that demonstrates a robust methyltransferase activity. Using a variety of substrates, we demonstrate that the enzyme only catalyzes the formation of ω-monomethylarginine residues, and we confirm its activity as the prototype type III protein arginine methyltransferase. This enzyme is active on all recombinant human core histones, but histone H2B is a highly preferred substrate. Analysis of the specific methylation sites within intact histone H2B and within H2B and H4 peptides revealed novel post-translational modification sites and a unique specificity of PRMT7 for methylating arginine residues in lysine- and arginine-rich regions. We demonstrate that a prominent substrate recognition motif consists of a pair of arginine residues separated by one residue (RXR motif). These findings will significantly accelerate substrate profile analysis, biological function study, and inhibitor discovery for PRMT7.
Keywords: Histone Methylation; Mass Spectrometry (MS); Methyltransferases; Post-translational Modification; Protein Methylation; Protein-arginine Methyltransferases (PRMT); S-Adenosylmethionine (AdoMet).
Figures

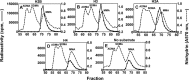

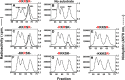


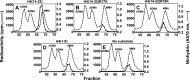

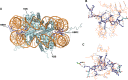
References
-
- Walsh C. T. (2006) Post-translational Modification of Proteins: Expanding Nature's Inventory, pp. 1–490, Roberts and Co. Publishers, Englewood, CO
-
- Yang Y., Bedford M. T. (2013) Protein-arginine methyltransferases and cancer. Nat. Rev. Cancer 13, 37–50 - PubMed
-
- Miranda T. B., Miranda M., Frankel A., Clarke S. (2004) PRMT7 is a member of the protein arginine methyltransferase family with a distinct substrate specificity. J. Biol. Chem. 279, 22902–22907 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

