The diarylheptanoid hirsutenone sensitizes chemoresistant ovarian cancer cells to cisplatin via modulation of apoptosis-inducing factor and X-linked inhibitor of apoptosis
- PMID: 24247248
- PMCID: PMC3894349
- DOI: 10.1074/jbc.M113.513879
The diarylheptanoid hirsutenone sensitizes chemoresistant ovarian cancer cells to cisplatin via modulation of apoptosis-inducing factor and X-linked inhibitor of apoptosis
Abstract
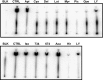
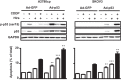
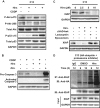
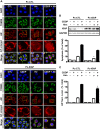
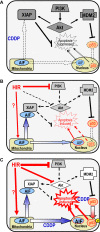
Cisplatin (CDDP) and its derivatives are considered first-line treatments for ovarian cancer (OVCA). However, despite initial results that often appear promising, in most cases patients will return with recurrent disease that fails to respond to further chemotherapy. We assayed a number of food phytochemicals with reported PI3K inhibitory ability to identify candidates that can influence CDDP treatment outcomes in chemoresistant OVCA cell lines. A direct comparison revealed that the diarylheptanoid hirsutenone from the tree bark of Alnus hirsuta var. sibirica was superior at inducing CDDP sensitivity in a number of chemoresistant cancer cell lines. Whereas hirsutenone treatment activated p53, its modest efficacy in p53-mutant and -null cell lines suggested the existence of a p53-independent mode of action. Further investigation revealed that hirsutenone causes CDDP-dependent apoptosis in chemoresistant cells by ubiquitin-proteasome-dependent X-linked inhibitor of apoptosis degradation and by enhancing the translocation of apoptosis-inducing factor from the mitochondria to the nucleus. This was found to be, at least in part, under the influence of upstream Akt activity, linking hirsutenone-dependent PI3K inhibition with downstream effects on apoptosis-inducing factor, X-linked inhibitor of apoptosis, and apoptosis. Our findings provide rationale for further investigation of the effects of hirsutenone on chemoresistant OVCA in clinical studies.
Keywords: AIF; Akt; Chemoresistance; Hirsutenone; Ovarian Cancer; XIAP; p53.
Figures



Similar articles
-
Piceatannol enhances cisplatin sensitivity in ovarian cancer via modulation of p53, X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial fission.J Biol Chem. 2013 Aug 16;288(33):23740-50. doi: 10.1074/jbc.M113.487686. Epub 2013 Jul 5. J Biol Chem. 2013. PMID: 23833193 Free PMC article.
-
Calpain-mediated processing of p53-associated parkin-like cytoplasmic protein (PARC) affects chemosensitivity of human ovarian cancer cells by promoting p53 subcellular trafficking.J Biol Chem. 2012 Feb 3;287(6):3963-75. doi: 10.1074/jbc.M111.314765. Epub 2011 Nov 23. J Biol Chem. 2012. PMID: 22117079 Free PMC article.
-
Diarylheptanoid hirsutenone enhances apoptotic effect of TRAIL on epithelial ovarian carcinoma cell lines via activation of death receptor and mitochondrial pathway.Invest New Drugs. 2012 Apr;30(2):548-57. doi: 10.1007/s10637-010-9601-5. Epub 2010 Dec 1. Invest New Drugs. 2012. PMID: 21120579
-
Role of X-linked inhibitor of apoptosis protein in chemoresistance in ovarian cancer: possible involvement of the phosphoinositide-3 kinase/Akt pathway.Drug Resist Updat. 2002 Jul-Aug;5(3-4):131-46. doi: 10.1016/s1368-7646(02)00003-1. Drug Resist Updat. 2002. PMID: 12237081 Review.
-
Targeting Mitochondria for Treatment of Chemoresistant Ovarian Cancer.Int J Mol Sci. 2019 Jan 8;20(1):229. doi: 10.3390/ijms20010229. Int J Mol Sci. 2019. PMID: 30626133 Free PMC article. Review.
Cited by
-
Akt+ IKKα/β+ Rab5+ Signalosome Mediate the Endosomal Recruitment of Sec61 and Contribute to Cross-Presentation in Bone Marrow Precursor Cells.Vaccines (Basel). 2020 Sep 17;8(3):539. doi: 10.3390/vaccines8030539. Vaccines (Basel). 2020. PMID: 32957586 Free PMC article.
-
Antioxidative and anticancer effects of Tacca chantrieri extract enhancing cisplatin sensitivity in cholangiocarcinoma cells.PLoS One. 2025 Jan 16;20(1):e0317111. doi: 10.1371/journal.pone.0317111. eCollection 2025. PLoS One. 2025. PMID: 39820585 Free PMC article.
-
Safety and Efficacy in Advanced Solid Tumors of a Targeted Nanocomplex Carrying the p53 Gene Used in Combination with Docetaxel: A Phase 1b Study.Mol Ther. 2016 Sep;24(9):1697-706. doi: 10.1038/mt.2016.135. Epub 2016 Jun 30. Mol Ther. 2016. PMID: 27357628 Free PMC article. Clinical Trial.
-
Identification of Chaetocin as a Potent non-ROS-mediated Anticancer Drug Candidate for Gastric Cancer.J Cancer. 2019 Jun 9;10(16):3678-3690. doi: 10.7150/jca.32803. eCollection 2019. J Cancer. 2019. PMID: 31333785 Free PMC article.
-
Apoptosis-inducing factor: a mitochondrial protein associated with metabolic diseases-a narrative review.Cardiovasc Diagn Ther. 2023 Jun 30;13(3):609-622. doi: 10.21037/cdt-23-123. Epub 2023 Jun 19. Cardiovasc Diagn Ther. 2023. PMID: 37405018 Free PMC article. Review.
References
-
- American Cancer Society (2012) Cancer Facts and Figures 2012. American Cancer Society, Atlanta, GA
-
- Covens A., Carey M., Bryson P., Verma S., Fung Kee Fung M., Johnston M. (2002) Systematic review of first-line chemotherapy for newly diagnosed postoperative patients with stage II, III, or IV epithelial ovarian cancer. Gynecol. Oncol. 85, 71–80 - PubMed
-
- Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T. (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

