Chaperones GroEL/GroES accelerate the refolding of a multidomain protein through modulating on-pathway intermediates
- PMID: 24247249
- PMCID: PMC3879552
- DOI: 10.1074/jbc.M113.518373
Chaperones GroEL/GroES accelerate the refolding of a multidomain protein through modulating on-pathway intermediates
Abstract
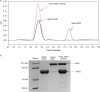
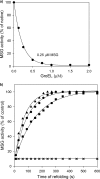
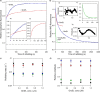
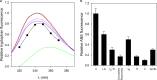
Despite a vast amount information on the interplay of GroEL, GroES, and ATP in chaperone-assisted folding, the molecular details on the conformational dynamics of folding polypeptide during its GroEL/GroES-assisted folding cycle is quite limited. Practically no such studies have been reported to date on large proteins, which often have difficulty folding in vitro. The effect of the GroEL/GroES chaperonin system on the folding pathway of an 82-kDa slow folding protein, malate synthase G (MSG), was investigated. GroEL bound to the burst phase intermediate of MSG and accelerated the slowest kinetic phase associated with the formation of native topology in the spontaneous folding pathway. GroEL slowly induced conformational changes on the bound burst phase intermediate, which was then transformed into a more folding-compatible form. Subsequent addition of ATP or GroES/ATP to the GroEL-MSG complex led to the formation of the native state via a compact intermediate with the rate several times faster than that of spontaneous refolding. The presence of GroES doubled the ATP-dependent reactivation rate of bound MSG by preventing multiple cycles of its GroEL binding and release. Because GroES bound to the trans side of GroEL-MSG complex, it may be anticipated that confinement of the substrate underneath the co-chaperone is not required for accelerating the rate in the assisted folding pathway. The potential role of GroEL/GroES in assisted folding is most likely to modulate the conformation of MSG intermediates that can fold faster and thereby eliminate the possibility of partial aggregation caused by the slow folding intermediates during its spontaneous refolding pathway.
Keywords: Aggregation; Folding Intermediates; GroEL; Large Protein Folding; Malate Synthase G; Molecular Chaperone; Protein Conformation; Protein Dynamics; Protein Folding; Refolding Kinetics.
Figures



Similar articles
-
Revisiting the GroEL-GroES reaction cycle via the symmetric intermediate implied by novel aspects of the GroEL(D398A) mutant.J Biol Chem. 2008 Aug 29;283(35):23774-81. doi: 10.1074/jbc.M802542200. Epub 2008 Jun 20. J Biol Chem. 2008. PMID: 18567584 Free PMC article.
-
Effective ATPase activity and moderate chaperonin-cochaperonin interaction are important for the functional single-ring chaperonin system.Biochem Biophys Res Commun. 2015 Oct 9;466(1):15-20. doi: 10.1016/j.bbrc.2015.08.034. Epub 2015 Aug 11. Biochem Biophys Res Commun. 2015. PMID: 26271593
-
Repetitive protein unfolding by the trans ring of the GroEL-GroES chaperonin complex stimulates folding.J Biol Chem. 2013 Oct 25;288(43):30944-55. doi: 10.1074/jbc.M113.480178. Epub 2013 Sep 10. J Biol Chem. 2013. PMID: 24022487 Free PMC article.
-
Molecular chaperone GroEL/ES: unfolding and refolding processes.Biochemistry (Mosc). 2013 Dec;78(13):1405-14. doi: 10.1134/S0006297913130038. Biochemistry (Mosc). 2013. PMID: 24490731 Review.
-
GroEL and the GroEL-GroES Complex.Subcell Biochem. 2017;83:483-504. doi: 10.1007/978-3-319-46503-6_17. Subcell Biochem. 2017. PMID: 28271487 Review.
Cited by
-
Spontaneous refolding of the large multidomain protein malate synthase G proceeds through misfolding traps.J Biol Chem. 2018 Aug 24;293(34):13270-13283. doi: 10.1074/jbc.RA118.003903. Epub 2018 Jun 29. J Biol Chem. 2018. PMID: 29959230 Free PMC article.
-
Role of chaperones and ATP synthase in DNA gyrase reactivation in Escherichia coli stationary-phase cells after nutrient addition.Springerplus. 2014 Nov 6;3:656. doi: 10.1186/2193-1801-3-656. eCollection 2014. Springerplus. 2014. PMID: 25485196 Free PMC article.
-
GroEL/ES mediated the in vivo recovery of TRAIL inclusion bodies in Escherichia coli.Sci Rep. 2018 Oct 25;8(1):15766. doi: 10.1038/s41598-018-34090-7. Sci Rep. 2018. PMID: 30361617 Free PMC article.
-
Dynamic Complexes in the Chaperonin-Mediated Protein Folding Cycle.Front Mol Biosci. 2016 Dec 8;3:80. doi: 10.3389/fmolb.2016.00080. eCollection 2016. Front Mol Biosci. 2016. PMID: 28008398 Free PMC article. Review.
-
Limited Diversity of Thermal Adaptation to a Critical Temperature in Zymomonas mobilis: Evidence from Multiple-Parallel Laboratory Evolution Experiments.Int J Mol Sci. 2025 Mar 26;26(7):3052. doi: 10.3390/ijms26073052. Int J Mol Sci. 2025. PMID: 40243698 Free PMC article.
References
-
- Horwich A. L., Farr G. W., Fenton W. A. (2006) GroEL-GroES-mediated protein folding. Chem. Rev. 106, 1917–1930 - PubMed
-
- Horwich A. L., Fenton W. A., Chapman E., Farr G. W. (2007) Two families of chaperonin: physiology and mechanism. Annu. Rev. Cell Dev. Biol. 23, 115–145 - PubMed
-
- Noivirt-Brik O., Unger R., Horovitz A. (2007) Low folding propensity and high translation efficiency distinguish in vivo substrates of GroEL from other Escherichia coli protein. Bioinformatics 23, 3276–3279 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

