Patterns of breast magnetic resonance imaging use in community practice
- PMID: 24247555
- PMCID: PMC3905972
- DOI: 10.1001/jamainternmed.2013.11963
Patterns of breast magnetic resonance imaging use in community practice
Abstract
Importance: Breast magnetic resonance imaging (MRI) is increasingly used for breast cancer screening, diagnostic evaluation, and surveillance. However, we lack data on national patterns of breast MRI use in community practice.
Objective: To describe patterns of breast MRI use in US community practice during the period 2005 through 2009.
Design, setting, and participants: Observational cohort study using data collected from 2005 through 2009 on breast MRI and mammography from 5 national Breast Cancer Surveillance Consortium registries. Data included 8931 breast MRI examinations and 1,288,924 screening mammograms from women aged 18 to 79 years.
Main outcomes and measures: We calculated the rate of breast MRI examinations per 1000 women with breast imaging within the same year and described the clinical indications for the breast MRI examinations by year and age. We compared women screened with breast MRI to women screened with mammography alone for patient characteristics and lifetime breast cancer risk.
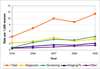
Results: The overall rate of breast MRI from 2005 through 2009 nearly tripled from 4.2 to 11.5 examinations per 1000 women, with the most rapid increase from 2005 to 2007 (P = .02). The most common clinical indication was diagnostic evaluation (40.3%), followed by screening (31.7%). Compared with women who received screening mammography alone, women who underwent screening breast MRI were more likely to be younger than 50 years, white non-Hispanic, and nulliparous and to have a personal history of breast cancer, a family history of breast cancer, and extremely dense breast tissue (all P < .001). The proportion of women screened using breast MRI at high lifetime risk for breast cancer (>20%) increased during the study period from 9% in 2005 to 29% in 2009.
Conclusions and relevance: Use of breast MRI for screening in high-risk women is increasing. However, our findings suggest that there is a need to improve appropriate use, including among women who may benefit from screening breast MRI.
Conflict of interest statement
Conflict of interest disclosure: Dr. Lehman has been a paid consultant to GE Healthcare, Bayer Healthcare, and Philips Healthcare. All other authors have no conflicts of interest to disclose.
Figures
Comment in
-
Patterns of breast magnetic resonance imaging use: an opportunity for data-driven resource allocation.JAMA Intern Med. 2014 Jan;174(1):122-4. doi: 10.1001/jamainternmed.2013.10502. JAMA Intern Med. 2014. PMID: 24247170 No abstract available.
References
-
- U.S. Food and Drug Administration. [Accessed 10/9/2012];MQSA National Statistics. http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandard....
-
- Howlader N, Noone AM, Krapcho M, et al. [Accessed May 12, 2012];SEER Cancer Statistics Review, 1975–2008, based on November 2010 SEER data submission, posted to the SEER web site, 2011. 2011 http://seer.cancer.gov/csr/1975_2008/
-
- Elmore L, Margenthaler JA. Breast MRI surveillance in women with prior curative-intent therapy for breast cancer. J Surg Res. 2010 Sep;163(1):58–62. - PubMed
-
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010 Mar-Apr;60(2):99–119. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 CA063740/CA/NCI NIH HHS/United States
- U01CA69976/CA/NCI NIH HHS/United States
- U01 CA070040/CA/NCI NIH HHS/United States
- RC2CA148577/CA/NCI NIH HHS/United States
- U01CA70013/CA/NCI NIH HHS/United States
- U01 CA086082/CA/NCI NIH HHS/United States
- P01 CA154292/CA/NCI NIH HHS/United States
- RC2 CA148577/CA/NCI NIH HHS/United States
- U01CA63736/CA/NCI NIH HHS/United States
- U01CA86082/CA/NCI NIH HHS/United States
- K12 HS019482/HS/AHRQ HHS/United States
- U01CA70040/CA/NCI NIH HHS/United States
- U01CA63740/CA/NCI NIH HHS/United States
- U01 CA063731/CA/NCI NIH HHS/United States
- U01 CA086076/CA/NCI NIH HHS/United States
- U01 CA069976/CA/NCI NIH HHS/United States
- U01CA86076/CA/NCI NIH HHS/United States
- U01 CA063736/CA/NCI NIH HHS/United States
- U01 CA070013/CA/NCI NIH HHS/United States
- U01CA63731/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical