Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial
- PMID: 24247587
- PMCID: PMC4111133
- DOI: 10.1001/jama.2013.282909
Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial
Abstract
Importance: Whether culture-expanded mesenchymal stem cells or whole bone marrow mononuclear cells are safe and effective in chronic ischemic cardiomyopathy is controversial.
Objective: To demonstrate the safety of transendocardial stem cell injection with autologous mesenchymal stem cells (MSCs) and bone marrow mononuclear cells (BMCs) in patients with ischemic cardiomyopathy.
Design, setting, and patients: A phase 1 and 2 randomized, blinded, placebo-controlled study involving 65 patients with ischemic cardiomyopathy and left ventricular (LV) ejection fraction less than 50% (September 1, 2009-July 12, 2013). The study compared injection of MSCs (n=19) with placebo (n = 11) and BMCs (n = 19) with placebo (n = 10), with 1 year of follow-up.
Interventions: Injections in 10 LV sites with an infusion catheter.
Main outcomes and measures: Treatment-emergent 30-day serious adverse event rate defined as a composite of death, myocardial infarction, stroke, hospitalization for worsening heart failure, perforation, tamponade, or sustained ventricular arrhythmias.
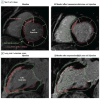
Results: No patient had a treatment-emergent serious adverse events at day 30. The 1-year incidence of serious adverse events was 31.6% (95% CI, 12.6% to 56.6%) for MSCs, 31.6% (95% CI, 12.6%-56.6%) for BMCs, and 38.1% (95% CI, 18.1%-61.6%) for placebo. Over 1 year, the Minnesota Living With Heart Failure score improved with MSCs (-6.3; 95% CI, -15.0 to 2.4; repeated measures of variance, P=.02) and with BMCs (-8.2; 95% CI, -17.4 to 0.97; P=.005) but not with placebo (0.4; 95% CI, -9.45 to 10.25; P=.38). The 6-minute walk distance increased with MSCs only (repeated measures model, P = .03). Infarct size as a percentage of LV mass was reduced by MSCs (-18.9%; 95% CI, -30.4 to -7.4; within-group, P = .004) but not by BMCs (-7.0%; 95% CI, -15.7% to 1.7%; within-group, P = .11) or placebo (-5.2%; 95% CI, -16.8% to 6.5%; within-group, P = .36). Regional myocardial function as peak Eulerian circumferential strain at the site of injection improved with MSCs (-4.9; 95% CI, -13.3 to 3.5; within-group repeated measures, P = .03) but not BMCs (-2.1; 95% CI, -5.5 to 1.3; P = .21) or placebo (-0.03; 95% CI, -1.9 to 1.9; P = .14). Left ventricular chamber volume and ejection fraction did not change.
Conclusions and relevance: Transendocardial stem cell injection with MSCs or BMCs appeared to be safe for patients with chronic ischemic cardiomyopathy and LV dysfunction. Although the sample size and multiple comparisons preclude a definitive statement about safety and clinical effect, these results provide the basis for larger studies to provide definitive evidence about safety and to assess efficacy of this new therapeutic approach.
Trial registration: clinicaltrials.gov Identifier: NCT00768066.
Conflict of interest statement
The other authors report no conflict of interest relevant to this work.
Figures




References
-
- Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355(12):1222–1232. - PubMed
-
- de la Fuente LM, Stertzer SH, Argentieri J, et al. Transendocardial autologous bone marrow in chronic myocardial infarction using a helical needle catheter: 1-year follow-up in an open-label, nonrandomized, single-center pilot study (the TABMMI study) Am Heart J. 2007;154(1):79–77. - PubMed
-
- Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114(1 Suppl):I101–I107. - PubMed
-
- Leistner DM, Fischer-Rasokat U, Honold J, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCAREAMI): final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol. 2011;100(10):925–934. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

