Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle
- PMID: 24247980
- PMCID: PMC3922499
- DOI: 10.1113/jphysiol.2013.266338
Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle
Abstract
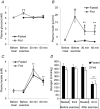
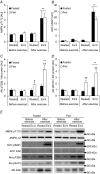
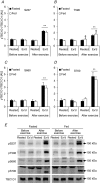
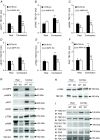
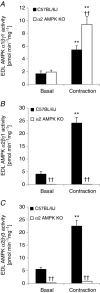
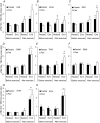
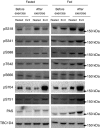
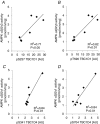
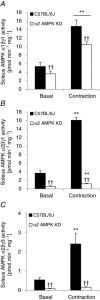
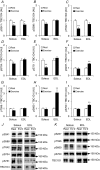
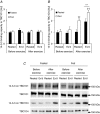
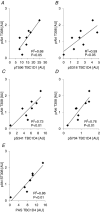
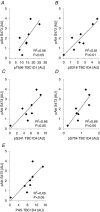
We investigated the phosphorylation signatures of two Rab-GTPase activating proteins TBC1D1 and TBC1D4 in human skeletal muscle in response to physical exercise and physiological insulin levels induced by a carbohydrate rich meal using a paired experimental design. Eight healthy male volunteers exercised in the fasted or fed state and muscle biopsies were taken before and immediately after exercise. We identified TBC1D1/4 phospho-sites that (1) did not respond to exercise or postprandial increase in insulin (TBC1D4: S666), (2) responded to insulin only (TBC1D4: S318), (3) responded to exercise only (TBC1D1: S237, S660, S700; TBC1D4: S588, S751), and (4) responded to both insulin and exercise (TBC1D1: T596; TBC1D4: S341, T642, S704). In the insulin-stimulated leg, Akt phosphorylation of both T308 and S473 correlated significantly with multiple sites on both TBC1D1 (T596) and TBC1D4 (S318, S341, S704). Interestingly, in the exercised leg in the fasted state TBC1D1 phosphorylation (S237, T596) correlated significantly with the activity of the α2/β2/γ3 AMPK trimer, whereas TBC1D4 phosphorylation (S341, S704) correlated with the activity of the α2/β2/γ1 AMPK trimer. Our data show differential phosphorylation of TBC1D1 and TBC1D4 in response to physiological stimuli in human skeletal muscle and support the idea that Akt and AMPK are upstream kinases. TBC1D1 phosphorylation signatures were comparable between in vitro contracted mouse skeletal muscle and exercised human muscle, and we show that AMPK regulated phosphorylation of these sites in mouse muscle. Contraction and exercise elicited a different phosphorylation pattern of TBC1D4 in mouse compared with human muscle, and although different circumstances in our experimental setup may contribute to this difference, the observation exemplifies that transferring findings between species is problematic.
Figures


Comment in
-
Let's get real about the regulation of TBC1D1 and TBC1D4 phosphorylation in skeletal muscle.J Physiol. 2014 Jan 15;592(2):253-4. doi: 10.1113/jphysiol.2013.269092. J Physiol. 2014. PMID: 24453349 Free PMC article. No abstract available.
References
-
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. - PubMed
-
- Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E1191–E1200. - PubMed
-
- Biensø RS, Ringholm S, Kiilerich K, Aachmann-Andersen NJ, Krogh-Madsen R, Guerra B, Plomgaard P, van HG, Treebak JT, Saltin B, Lundby C, Calbet JA, Pilegaard H, Wojtaszewski JF. GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance. Diabetes. 2012;61:1090–1099. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

