Dimerization of LTβR by LTα1β2 is necessary and sufficient for signal transduction
- PMID: 24248355
- PMCID: PMC3856818
- DOI: 10.1073/pnas.1310838110
Dimerization of LTβR by LTα1β2 is necessary and sufficient for signal transduction
Abstract
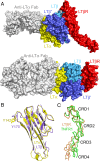
Homotrimeric TNF superfamily ligands signal by inducing trimers of their cognate receptors. As a biologically active heterotrimer, Lymphotoxin(LT)α1β2 is unique in the TNF superfamily. How the three unique potential receptor-binding interfaces in LTα1β2 trigger signaling via LTβ Receptor (LTβR) resulting in lymphoid organogenesis and propagation of inflammatory signals is poorly understood. Here we show that LTα1β2 possesses two binding sites for LTβR with distinct affinities and that dimerization of LTβR by LTα1β2 is necessary and sufficient for signal transduction. The crystal structure of a complex formed by LTα1β2, LTβR, and the fab fragment of an antibody that blocks LTβR activation reveals the lower affinity receptor-binding site. Mutations targeting each potential receptor-binding site in an engineered single-chain variant of LTα1β2 reveal the high-affinity site. NF-κB reporter assays further validate that disruption of receptor interactions at either site is sufficient to prevent signaling via LTβR.
Keywords: biophysics; crystallography; cytokines; mechanism.
Conflict of interest statement
Conflict of interest statement: All authors are employees of Genentech, Inc.
Figures


Similar articles
-
Lymphotoxin-alpha 1 beta 2 and LIGHT induce classical and noncanonical NF-kappa B-dependent proinflammatory gene expression in vascular endothelial cells.J Immunol. 2008 Mar 1;180(5):3467-77. doi: 10.4049/jimmunol.180.5.3467. J Immunol. 2008. PMID: 18292573 Free PMC article.
-
Lymphotoxin β receptor-mediated NFκB signaling promotes glial lineage differentiation and inhibits neuronal lineage differentiation in mouse brain neural stem/progenitor cells.J Neuroinflammation. 2018 Feb 20;15(1):49. doi: 10.1186/s12974-018-1074-z. J Neuroinflammation. 2018. PMID: 29463313 Free PMC article.
-
Lymphotoxin-β receptor activation by lymphotoxin-α(1)β(2) and LIGHT promotes tumor growth in an NFκB-dependent manner.Int J Cancer. 2011 Mar 15;128(6):1363-70. doi: 10.1002/ijc.25456. Int J Cancer. 2011. PMID: 20473944
-
Biology and signal transduction pathways of the Lymphotoxin-αβ/LTβR system.Cytokine Growth Factor Rev. 2011 Oct-Dec;22(5-6):301-10. doi: 10.1016/j.cytogfr.2011.11.007. Cytokine Growth Factor Rev. 2011. PMID: 22152226 Review.
-
Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways.Immunol Rev. 2008 Jun;223:186-201. doi: 10.1111/j.1600-065X.2008.00629.x. Immunol Rev. 2008. PMID: 18613837 Free PMC article. Review.
Cited by
-
Membrane lymphotoxin-α2β is a novel tumor necrosis factor (TNF) receptor 2 (TNFR2) agonist.Cell Death Dis. 2021 Apr 6;12(4):360. doi: 10.1038/s41419-021-03633-8. Cell Death Dis. 2021. PMID: 33824270 Free PMC article.
-
The Role of Interleukin-7 in the Formation of Tertiary Lymphoid Structures and Their Prognostic Value in Gastrointestinal Cancers.J Immunother Precis Oncol. 2022 Oct 26;5(4):105-117. doi: 10.36401/JIPO-22-10. eCollection 2022 Nov. J Immunother Precis Oncol. 2022. PMID: 36483588 Free PMC article. Review.
-
Role of the Lymphotoxin/LIGHT System in the Development and Maintenance of Reticular Networks and Vasculature in Lymphoid Tissues.Front Immunol. 2014 Feb 11;5:47. doi: 10.3389/fimmu.2014.00047. eCollection 2014. Front Immunol. 2014. PMID: 24575096 Free PMC article. Review.
-
Evaluating the Use of Antibody Variable Region (Fv) Charge as a Risk Assessment Tool for Predicting Typical Cynomolgus Monkey Pharmacokinetics.J Biol Chem. 2015 Dec 11;290(50):29732-41. doi: 10.1074/jbc.M115.692434. Epub 2015 Oct 21. J Biol Chem. 2015. PMID: 26491012 Free PMC article.
-
TNFSF14-Derived Molecules as a Novel Treatment for Obesity and Type 2 Diabetes.Int J Mol Sci. 2021 Sep 30;22(19):10647. doi: 10.3390/ijms221910647. Int J Mol Sci. 2021. PMID: 34638990 Free PMC article.
References
-
- Browning JL, et al. Preparation and characterization of soluble recombinant heterotrimeric complexes of human lymphotoxins alpha and beta. J Biol Chem. 1996;271(15):8618–8626. - PubMed
-
- Ware CF. Network communications: Lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. - PubMed
-
- Gommerman JL, Browning JL. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nat Rev Immunol. 2003;3(8):642–655. - PubMed
-
- Tumanov AV, Kuprash DV, Nedospasov SA. The role of lymphotoxin in development and maintenance of secondary lymphoid tissues. Cytokine Growth Factor Rev. 2003;14(3-4):275–288. - PubMed
-
- Grogan JL, Ouyang WJ. A role for Th17 cells in the regulation of tertiary lymphoid follicles. Eur J Immunol. 2012;42(9):2255–2262. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

