Estimating functional connectivity in an electrically coupled interneuron network
- PMID: 24248377
- PMCID: PMC3856846
- DOI: 10.1073/pnas.1310983110
Estimating functional connectivity in an electrically coupled interneuron network
Abstract
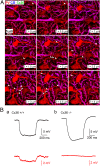
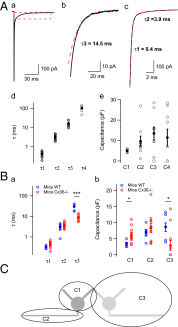
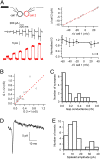
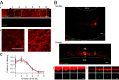
Even though it has been known for some time that in many mammalian brain areas interneurons are electrically coupled, a quantitative description of the network electrical connectivity and its impact on cellular passive properties is still lacking. Approaches used so far to solve this problem are limited because they do not readily distinguish junctions among direct neighbors from indirect junctions involving intermediary, multiply connected cells. In the cerebellar cortex, anatomical and functional evidence indicates electrical coupling between molecular layer interneurons (basket and stellate cells). An analysis of the capacitive currents obtained under voltage clamp in molecular layer interneurons of juvenile rats or mice reveals an exponential component with a time constant of ~20 ms, which represents capacitive loading of neighboring cells through gap junctions. These results, taken together with dual cell recording of electrical synapses, have led us to estimate the number of direct neighbors to be ~4 for rat basket cells and ~1 for rat stellate cells. The weighted number of neighbors (number of neighbors, both direct and indirect, weighted with the percentage of voltage deflection at steady state) was 1.69 in basket cells and 0.23 in stellate cells. The last numbers indicate the spread of potential changes in the network and serve to estimate the contribution of gap junctions to cellular input conductance. In conclusion the present work offers effective tools to analyze the connectivity of electrically connected interneuron networks, and it indicates that in juvenile rodents, electrical communication is stronger among basket cells than among stellate cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci. 2001;2(6):425–433. - PubMed
-
- Tamás G, Buhl EH, Lörincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3(4):366–371. - PubMed
-
- Hormuzdi SG, et al. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31(3):487–495. - PubMed
-
- Lewis TJ, Rinzel J. Dynamics of spiking neurons connected by both inhibitory and electrical coupling. J Comput Neurosci. 2003;14(3):283–309. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

