Grb2 promotes integrin-induced focal adhesion kinase (FAK) autophosphorylation and directs the phosphorylation of protein tyrosine phosphatase α by the Src-FAK kinase complex
- PMID: 24248601
- PMCID: PMC3911518
- DOI: 10.1128/MCB.00825-13
Grb2 promotes integrin-induced focal adhesion kinase (FAK) autophosphorylation and directs the phosphorylation of protein tyrosine phosphatase α by the Src-FAK kinase complex
Abstract
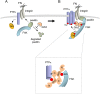
The integrin-activated Src-focal adhesion kinase (FAK) kinase complex phosphorylates PTPα at Tyr789, initiating PTPα-mediated signaling that promotes cell migration. Recruitment of the BCAR3-Cas complex by PTPα-phospho-Tyr789 at focal adhesions is one mechanism of PTPα signaling. The adaptor protein Grb2 is also recruited by PTPα-phospho-Tyr789, although the role of the PTPα-Grb2 complex in integrin signaling is unknown. We show that silencing Grb2 expression in fibroblasts abolishes PTPα-Tyr789 phosphorylation and that this is due to two unexpected actions of Grb2. First, Grb2 promotes integrin-induced autophosphorylation of FAK-Tyr397. This is impaired in Grb2-depleted cells and prohibits FAK activation and formation of the Src-FAK complex. Grb2-depleted cells contain less paxillin, and paxillin overexpression rescues FAK-Tyr397 phosphorylation, suggesting that the FAK-activating action of Grb2 involves paxillin. A second distinct role for Grb2 in PTPα-Tyr789 phosphorylation involves Grb2-mediated coupling of Src-FAK and PTPα. This requires two phosphosites, FAK-Tyr925 and PTPα-Tyr789, for Grb2-Src homology 2 (SH2) binding. We propose that a Grb2 dimer links FAK and PTPα, and this positions active Src-FAK in proximity with other, perhaps integrin-clustered, molecules of PTPα to enable maximal PTPα-Tyr789 phosphorylation. These findings identify Grb2 as a new FAK activator and reveal its essential role in coordinating PTPα tyrosine phosphorylation to enable downstream integrin signaling and migration.
Figures







Similar articles
-
Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is required for cytoskeletal reorganization and cell migration.J Biol Chem. 2006 Apr 28;281(17):11972-80. doi: 10.1074/jbc.M600561200. Epub 2006 Feb 28. J Biol Chem. 2006. PMID: 16507567
-
Protein tyrosine phosphatase α phosphotyrosyl-789 binds BCAR3 to position Cas for activation at integrin-mediated focal adhesions.Mol Cell Biol. 2012 Sep;32(18):3776-89. doi: 10.1128/MCB.00214-12. Epub 2012 Jul 16. Mol Cell Biol. 2012. PMID: 22801373 Free PMC article.
-
Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events.Mol Cell Biol. 1998 May;18(5):2571-85. doi: 10.1128/MCB.18.5.2571. Mol Cell Biol. 1998. PMID: 9566877 Free PMC article.
-
Role of focal adhesion kinase in integrin signaling.Int J Biochem Cell Biol. 1997 Aug-Sep;29(8-9):1085-96. doi: 10.1016/s1357-2725(97)00051-4. Int J Biochem Cell Biol. 1997. PMID: 9416004 Review.
-
Signaling through focal adhesion kinase.Bioessays. 1997 Feb;19(2):137-45. doi: 10.1002/bies.950190208. Bioessays. 1997. PMID: 9046243 Review.
Cited by
-
TGF-β2 induces Grb2 to recruit PI3-K to TGF-RII that activates JNK/AP-1-signaling and augments invasiveness of Theileria-transformed macrophages.Sci Rep. 2015 Oct 29;5:15688. doi: 10.1038/srep15688. Sci Rep. 2015. PMID: 26511382 Free PMC article.
-
c-CBL regulates melanoma proliferation, migration, invasion and the FAK-SRC-GRB2 nexus.Oncotarget. 2016 Aug 16;7(33):53869-53880. doi: 10.18632/oncotarget.10861. Oncotarget. 2016. PMID: 27472394 Free PMC article.
-
Targeting Focal Adhesion Kinase Using Inhibitors of Protein-Protein Interactions.Cancers (Basel). 2018 Aug 21;10(9):278. doi: 10.3390/cancers10090278. Cancers (Basel). 2018. PMID: 30134553 Free PMC article. Review.
-
SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1.Mol Cancer. 2014 Apr 29;13:95. doi: 10.1186/1476-4598-13-95. Mol Cancer. 2014. PMID: 24775912 Free PMC article.
-
Protein tyrosine phosphatases in cell adhesion.Biochem J. 2021 Mar 12;478(5):1061-1083. doi: 10.1042/BCJ20200511. Biochem J. 2021. PMID: 33710332 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
