Interaction of delta-like 1 homolog (Drosophila) with prohibitins and its impact on tumor cell clonogenicity
- PMID: 24249679
- PMCID: PMC3946965
- DOI: 10.1158/1541-7786.MCR-13-0360
Interaction of delta-like 1 homolog (Drosophila) with prohibitins and its impact on tumor cell clonogenicity
Abstract
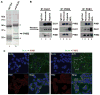
Cancer stem cell characteristics, especially their self-renewal and clonogenic potentials, play an essential role in malignant progression and response to anticancer therapies. Currently, it remains largely unknown what pathways are involved in the regulation of cancer cell stemness and differentiation. Previously, we found that delta-like 1 homolog (Drosophila) or DLK1, a developmentally regulated gene, plays a critical role in the regulation of differentiation, self-renewal, and tumorigenic growth of neuroblastoma cells. Here, we show that DLK1 specifically interacts with the prohibitin 1 (PHB1) and PHB2, two closely related genes with pleiotropic functions, including regulation of mitochondrial function and gene transcription. DLK1 interacts with the PHB1-PHB2 complex via its cytoplasmic domain and regulates mitochondrial functions, including mitochondrial membrane potential and production of reactive oxygen species. We have further found that PHB1 and especially PHB2 regulate cancer cell self-renewal as well as their clonogenic potential. Hence, the DLK1-PHB interaction constitutes a new signaling pathway that maintains clonogenicity and self-renewal potential of cancer cells.
Implications: This study provides a new mechanistic insight into the regulation of the stem cell characteristics of cancer cells.
©2013 AACR.
Conflict of interest statement
Disclosure of Potential Conflicts of Interests: The authors claim no potential conflicts of interests.
Figures
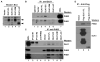



Similar articles
-
Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity.Cancer Res. 2009 Dec 15;69(24):9271-80. doi: 10.1158/0008-5472.CAN-09-1605. Cancer Res. 2009. PMID: 19934310 Free PMC article.
-
Effect of β-carotene on cancer cell stemness and differentiation in SK-N-BE(2)C neuroblastoma cells.Oncol Rep. 2013 Oct;30(4):1869-77. doi: 10.3892/or.2013.2643. Epub 2013 Jul 30. Oncol Rep. 2013. PMID: 23900747
-
DLK1, delta-like 1 homolog (Drosophila), regulates tumor cell differentiation in vivo.Cancer Lett. 2012 May 1;318(1):26-33. doi: 10.1016/j.canlet.2011.11.032. Epub 2011 Dec 3. Cancer Lett. 2012. PMID: 22142700 Free PMC article.
-
Essential Protein PHB2 and Its Regulatory Mechanisms in Cancer.Cells. 2023 Apr 21;12(8):1211. doi: 10.3390/cells12081211. Cells. 2023. PMID: 37190120 Free PMC article. Review.
-
Emerging Roles of DLK1 in the Stem Cell Niche and Cancer Stemness.J Histochem Cytochem. 2022 Jan;70(1):17-28. doi: 10.1369/00221554211048951. Epub 2021 Oct 4. J Histochem Cytochem. 2022. PMID: 34606325 Free PMC article. Review.
Cited by
-
DLK1 promoted ischemic angiogenesis through notch1 signaling in endothelial progenitor cells.Acta Pharmacol Sin. 2024 Dec;45(12):2553-2566. doi: 10.1038/s41401-024-01346-0. Epub 2024 Jul 26. Acta Pharmacol Sin. 2024. PMID: 39060522
-
Dlk1 maintains adult mice long-term HSCs by activating Notch signaling to restrict mitochondrial metabolism.Exp Hematol Oncol. 2023 Jan 18;12(1):11. doi: 10.1186/s40164-022-00369-9. Exp Hematol Oncol. 2023. PMID: 36653853 Free PMC article.
-
The imprinted gene Delta like non-canonical notch ligand 1 (Dlk1) associates with obesity and triggers insulin resistance through inhibition of skeletal muscle glucose uptake.EBioMedicine. 2019 Aug;46:368-380. doi: 10.1016/j.ebiom.2019.07.070. Epub 2019 Aug 3. EBioMedicine. 2019. PMID: 31383551 Free PMC article.
-
Silencing YTHDF2 Induces Apoptosis of Neuroblastoma Cells In a Cell Line-Dependent Manner via Regulating the Expression of DLK1.Mol Neurobiol. 2025 Jul;62(7):8121-8134. doi: 10.1007/s12035-025-04759-y. Epub 2025 Feb 20. Mol Neurobiol. 2025. PMID: 39979690
-
A novel c-Kit/phospho-prohibitin axis enhances ovarian cancer stemness and chemoresistance via Notch3-PBX1 and β-catenin-ABCG2 signaling.J Biomed Sci. 2020 Mar 13;27(1):42. doi: 10.1186/s12929-020-00638-x. J Biomed Sci. 2020. PMID: 32169072 Free PMC article.
References
-
- Laborda J. The role of the epidermal growth factor-like protein dlk in cell differentiation. Histol Histopathol. 2000;15:119–29. - PubMed
-
- Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, et al. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. - PubMed
-
- Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–34. - PubMed
-
- Tornehave D, Jensen CH, Teisner B, Larsson LI. FA1 immunoreactivity in endocrine tumours and during development of the human fetal pancreas; negative correlation with glucagon expression. Histochem Cell Biol. 1996;106:535–42. - PubMed
-
- Van Limpt VA, Chan AJ, Van Sluis PG, Caron HN, Van Noesel CJ, Versteeg R. High delta-like 1 expression in a subset of neuroblastoma cell lines corresponds to a differentiated chromaffin cell type. Int J Cancer. 2003;105:61–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

