Growth properties of cardiac stem cells are a novel biomarker of patients' outcome after coronary bypass surgery
- PMID: 24249720
- PMCID: PMC3969331
- DOI: 10.1161/CIRCULATIONAHA.113.006591
Growth properties of cardiac stem cells are a novel biomarker of patients' outcome after coronary bypass surgery
Erratum in
- Circulation. 2014 Aug 12;130(7):e65
-
Correction.Circulation. 2015 Dec 1;132(22):e363. doi: 10.1161/CIR.0000000000000341. Circulation. 2015. PMID: 26621666 No abstract available.
Retraction in
-
Retraction of: Growth Properties of Cardiac Stem Cells Are a Novel Biomarker of Patients' Outcome After Coronary Bypass Surgery.Circulation. 2019 Feb 12;139(7):e40. doi: 10.1161/CIR.0000000000000642. Circulation. 2019. PMID: 30586779 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern.Circ Res. 2019 Jan 18;124(2):e4-e5. doi: 10.1161/RES.0000000000000241. Circ Res. 2019. PMID: 30582460 No abstract available.
-
Expression of Concern.Circulation. 2019 Jan 15;139(3):e5-e6. doi: 10.1161/CIR.0000000000000639. Circulation. 2019. PMID: 30615475 No abstract available.
Abstract
Background: The efficacy of bypass surgery in patients with ischemic cardiomyopathy is not easily predictable; preoperative clinical conditions may be similar, but the outcome may differ significantly. We hypothesized that the growth reserve of cardiac stem cells (CSCs) and circulating cytokines promoting CSC activation are critical determinants of ventricular remodeling in this patient population.
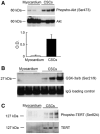
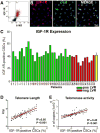
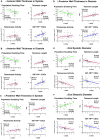
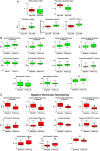
Methods and results: To document the growth kinetics of CSCs, population-doubling time, telomere length, telomerase activity, and insulin-like growth factor-1 receptor expression were measured in CSCs isolated from 38 patients undergoing bypass surgery. Additionally, the blood levels of insulin-like growth factor-1, hepatocyte growth factor, and vascular endothelial growth factor were evaluated. The variables of CSC growth were expressed as a function of the changes in wall thickness, chamber diameter and volume, ventricular mass-to-chamber volume ratio, and ejection fraction, before and 12 months after surgery. A high correlation was found between indices of CSC function and cardiac anatomy. Negative ventricular remodeling was not observed if CSCs retained a significant growth reserve. The high concentration of insulin-like growth factor-1 systemically pointed to the insulin-like growth factor-1-insulin-like growth factor-1 receptor system as a major player in the adaptive response of the myocardium. hepatocyte growth factor, a mediator of CSC migration, was also high in these patients preoperatively, as was vascular endothelial growth factor, possibly reflecting the vascular growth needed before bypass surgery. Conversely, a decline in CSC growth was coupled with wall thinning, chamber dilation, and depressed ejection fraction.
Conclusions: The telomere-telomerase axis, population-doubling time, and insulin-like growth factor-1 receptor expression in CSCs, together with a high circulating level of insulin-like growth factor-1, represent a novel biomarker able to predict the evolution of ischemic cardiomyopathy following revascularization.
Keywords: coronary artery disease; receptor, IGF type 1; stem cells; telomerase; telomere; ventricular remodeling.
Conflict of interest statement
Figures




Comment in
-
Predicting the future with stem cells.Circulation. 2014 Jan 14;129(2):136-8. doi: 10.1161/CIRCULATIONAHA.113.007045. Epub 2013 Nov 18. Circulation. 2014. PMID: 24249719 Free PMC article. No abstract available.
-
Letter by Li and Shen regarding article, "growth properties of cardiac stem cells are a novel biomarker of patients' outcome after coronary bypass surgery".Circulation. 2014 Sep 23;130(13):e117. doi: 10.1161/CIRCULATIONAHA.113.008500. Circulation. 2014. PMID: 25245851 No abstract available.
-
Response to letter regarding article, "growth properties of cardiac stem cells are a novel biomarker of patients' outcome after coronary bypass surgery".Circulation. 2014 Sep 23;130(13):e118-9. doi: 10.1161/CIRCULATIONAHA.114.010924. Circulation. 2014. PMID: 25245852 No abstract available.
References
-
- Rizzello V, Poldermans D, Boersma E, Biagini E, Schinkel AF, Krenning B, Elhendy A, Vourvouri EC, Sozzi FB, Maat A, Crea F, Roelandt JR, Bax JJ. Opposite patterns of left ventricular remodeling after coronary revascularization in patients with ischemic cardiomyopathy: role of myocardial viability. Circulation. 2004;110:2383–2388. - PubMed
-
- Schinkel AF, Poldermans D, Rizzello V, Vanoverschelde JL, Elhendy A, Boersma E, Roelandt JR, Bax JJ. Why do patients with ischemic cardiomyopathy and a substantial amount of viable myocardium not always recover in function after revascularization? J Thorac Cardiovasc Surg. 2004;127:385–390. - PubMed
-
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. - PubMed
-
- Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. - PubMed
-
- Verma A, Meris A, Skali H, Ghali JK, Arnold JM, Bourgoun M, Velazquez EJ, McMurray JJ, Kober L, Pfeffer MA, Califf RM, Solomon SD. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008;1:582–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

