Gabapentin Determination in Human Plasma and Capsule by Coupling of Solid Phase Extraction, Derivatization Reaction, and UV-Vis Spectrophotometry
- PMID: 24250630
- PMCID: PMC3813261
Gabapentin Determination in Human Plasma and Capsule by Coupling of Solid Phase Extraction, Derivatization Reaction, and UV-Vis Spectrophotometry
Abstract
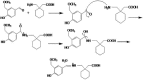
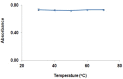
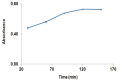
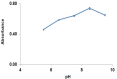
Gabapentin is an anticonvulsant widely used in the treatment of epilepsy. No peculiar chromophore is available on the gabapentin moiety for direct analysis by absorption spectrophotometry. A sensitive spectrophotometric method for the determination of gabapentin in bulk, pharmaceutical formulations and human plasma has been developed. In this method, gabapentin directly derivatized with vanillin and analyzed without any extraction in bulk and pharmaceutical dosage form and in plasma samples, it was extracted with a reversed-phase solid-phase extraction (SPE) cartridge followed by derivatization with vanillin. Analysis was performed by a spectrophotometer system. The quantitation limit of gabapentin in human plasma was 0.8 mg/L. The method was linear over the concentration range of 10.0-90.0 mg/L and 0.8-10.0 mg/L for pharmaceutical dosage form and plasma, respectively. The method was precise (relative standard deviation, RSD <1.20%) and accurate (relative mean error <5.5%) for both pharmaceutical dosage form and plasma samples. Mean absolute recoveries were 94.5% for plasma.
Keywords: Derivatization; Gabapentin; Plasma; SPE; Spectrophotometry; Vanillin.
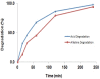
Figures
References
-
- Clarke H, Bonin RP, Orser BA, Englesakis M, Wijeysundera DN, Katz J. The prevention of chronic postsurgical pain using gabapentin and pregabalin: A combined systematic review and meta-analysis. Anesth. Analg. 2012;115:428–442. - PubMed
-
- Bahrami G, Mohammadi B. Sensitive microanalysis of gabapentin by high-performance liquid chromatography in human serum using pre-column derivatization with 4-chloro-7-nitrobenzofurazan: Application to a bioequivalence study. J. Chromatogr B. 2006;837:24–28. - PubMed
-
- Galande VR, Baheti K, Dehghan M. UV-Vis spectrophotometric method for estimation of Gabapentin and Methylcobalamin in bulk and tablet. Int. J. Chem.Tech. Res. 2010;2:695–699.
-
- Gujral RS, Haque SM, Shanker P. A Novel Quantitative Spectrophotometric Method for the Analysis of Gabapentin Hydrochloride. J. Pharm. Sci. Tech. 2010;2:222–229.
LinkOut - more resources
Full Text Sources
