Compact non-contact total emission detection for in vivo multiphoton excitation microscopy
- PMID: 24251437
- PMCID: PMC4132828
- DOI: 10.1111/jmi.12099
Compact non-contact total emission detection for in vivo multiphoton excitation microscopy
Abstract
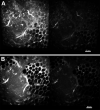
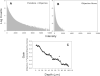
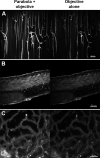
We describe a compact, non-contact design for a total emission detection (c-TED) system for intra-vital multiphoton imaging. To conform to a standard upright two-photon microscope design, this system uses a parabolic mirror surrounding a standard microscope objective in concert with an optical path that does not interfere with normal microscope operation. The non-contact design of this device allows for maximal light collection without disrupting the physiology of the specimen being examined. Tests were conducted on exposed tissues in live animals to examine the emission collection enhancement of the c-TED device compared to heavily optimized objective-based emission collection. The best light collection enhancement was seen from murine fat (5×-2× gains as a function of depth), whereas murine skeletal muscle and rat kidney showed gains of over two and just under twofold near the surface, respectively. Gains decreased with imaging depth (particularly in the kidney). Zebrafish imaging on a reflective substrate showed close to a twofold gain throughout the entire volume of an intact embryo (approximately 150 μm deep). Direct measurement of bleaching rates confirmed that the lower laser powers, enabled by greater light collection efficiency, yielded reduced photobleaching in vivo. The potential benefits of increased light collection in terms of speed of imaging and reduced photo-damage, as well as the applicability of this device to other multiphoton imaging methods is discussed.
Keywords: Imaging; light collection; two-photon microscopy.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Figures

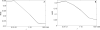

References
-
- Combs CA, Smirnov A, Chess D, McGavern DB, Schroeder JL, Riley J, Kang SS, Lugar-Hammer M, Gandjbakhche A, Knutson JR, Balaban RS. Optimizing multiphoton fluorescence microscopy light collection from living tissue by noncontact total emission detection (epiTED). J Microsc. 2011;241:153–161. - PMC - PubMed
-
- Combs CA, Smirnov AV, Riley JD, Gandjbakhche AH, Knutson JR, Balaban RS. Optimization of multiphoton excitation microscopy by total emission detection using a parabolic light reflector. J Microsc. 2007;228:330–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

