Noise-rearing disrupts the maturation of multisensory integration
- PMID: 24251451
- PMCID: PMC3944832
- DOI: 10.1111/ejn.12423
Noise-rearing disrupts the maturation of multisensory integration
Abstract
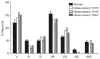
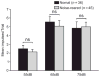
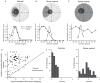
It is commonly believed that the ability to integrate information from different senses develops according to associative learning principles as neurons acquire experience with co-active cross-modal inputs. However, previous studies have not distinguished between requirements for co-activation versus co-variation. To determine whether cross-modal co-activation is sufficient for this purpose in visual-auditory superior colliculus (SC) neurons, animals were reared in constant omnidirectional noise. By masking most spatiotemporally discrete auditory experiences, the noise created a sensory landscape that decoupled stimulus co-activation and co-variance. Although a near-normal complement of visual-auditory SC neurons developed, the vast majority could not engage in multisensory integration, revealing that visual-auditory co-activation was insufficient for this purpose. That experience with co-varying stimuli is required for multisensory maturation is consistent with the role of the SC in detecting and locating biologically significant events, but it also seems likely that this is a general requirement for multisensory maturation throughout the brain.
Keywords: cat; cross-modal; hearing; vision.
© 2013 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures




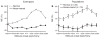


References
-
- Alvarado JC, Vaughan JW, Stanford TR, Stein BE. Multi-sensory versus unisensory integration: contrasting modes in the superior colliculus. J Neurophysiol. 2007b;97:3193–3205. - PubMed
-
- Carrasco MM, Razak KA, Pallas SL. Visual experience is necessary for maintenance but not development of receptive fields in superior colliculus. J Neurophysiol. 2005;94:1962–1970. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

