Mechanisms of epileptiform synchronization in cortical neuronal networks
- PMID: 24251567
- PMCID: PMC4880466
- DOI: 10.2174/0929867320666131119151136
Mechanisms of epileptiform synchronization in cortical neuronal networks
Abstract
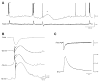
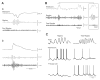
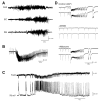
Neuronal synchronization supports different physiological states such as cognitive functions and sleep, and it is mirrored by identifiable EEG patterns ranging from gamma to delta oscillations. However, excessive neuronal synchronization is often the hallmark of epileptic activity in both generalized and partial epileptic disorders. Here, I will review the synchronizing mechanisms involved in generating epileptiform activity in the limbic system, which is closely involved in the pathophysiogenesis of temporal lobe epilepsy (TLE). TLE is often associated to a typical pattern of brain damage known as mesial temporal sclerosis, and it is one of the most refractory adult form of partial epilepsy. This epileptic disorder can be reproduced in animals by topical or systemic injection of pilocarpine or kainic acid, or by repetitive electrical stimulation; these procedures induce an initial status epilepticus and cause 1-4 weeks later a chronic condition of recurrent limbic seizures. Remarkably, a similar, seizure-free, latent period can be identified in TLE patients who suffered an initial insult in childhood and develop partial seizures in adolescence or early adulthood. Specifically, I will focus here on the neuronal mechanisms underlying three abnormal types of neuronal synchronization seen in both TLE patients and animal models mimicking this disorder: (i) interictal spikes; (ii) high frequency oscillations (80-500 Hz); and (iii) ictal (i.e., seizure) discharges. In addition, I will discuss the relationship between interictal spikes and ictal activity as well as recent evidence suggesting that specific seizure onsets in the pilocarpine model of TLE are characterized by distinctive patterns of spiking (also termed preictal) and high frequency oscillations.
Conflict of interest statement
The author(s) confirm that this article content has no conflicts of interest.
Figures


References
-
- Niedermeyer E, Lopes Da Silva FH. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins; 2005.
-
- Gloor P. The temporal lobe and limbic system. New York: Oxford University Press; 1997.
-
- Jefferys JGR, Jiruska P, de Curtis M, Avoli M. Limbic Network Synchronization and Temporal Lobe Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. - PubMed
-
- Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–930. - PMC - PubMed
-
- Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

