Differentiation of human adipose-derived stem cells into neuron-like cells which are compatible with photocurable three-dimensional scaffolds
- PMID: 24251600
- PMCID: PMC3993073
- DOI: 10.1089/ten.TEA.2012.0773
Differentiation of human adipose-derived stem cells into neuron-like cells which are compatible with photocurable three-dimensional scaffolds
Abstract
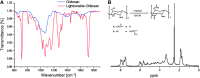
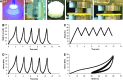
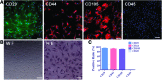
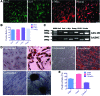
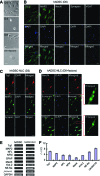
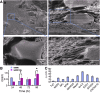
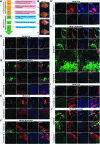
Multipotent human adipose-derived stromal/stem cells (hADSCs) hold a great promise for cell-based therapy for many devastating human diseases, such as spinal cord injury and stroke. If exogenous hADSCs can be cultured in a three-dimensional (3D) scaffold with effective proliferation and differentiation capacity, it will better mimic the in vivo environment, which will have profound impact on the therapeutic application of hADSCs. In this study, a group of elastic-dominant, porous bioscaffolds from photocurable chitosan and gelatin were fabricated and proven to be biocompatible with both hADSCs and hADSC-derived neuron-like cells (hADSC-NLCs) in vitro. The identity of harvested hADSCs was confirmed by their positive immunostaining of mesenchymal stem cell surface markers, CD29, CD44, and CD105, and also positive expression of stem markers, Sox-2, Oct-4, c-Myc, Nanog, and Klf4. Their multipotency was further confirmed by trilineage differentiation of hADSCs toward adipocyte, osteoblast, and chondrocyte. It was found that hADSCs could be conditioned to differentiate into neurons in vitro as determined by immunostaining the markers of Tuj1, MAP2, NeuN, and Synapsin. The hADSCs and hADSC-NLCs were proven to be biocompatible with 3D scaffold, which actually facilitated the proliferation and differentiation of hADSCs in vitro, by MTT assay and their neuronal gene expression profiling. Moreover, hADSC-NLCs, which were mixed with 3D scaffold and transplanted into traumatic brain injury mouse model, survived in vivo and led to the better repair of the damaged brain area. The immunohistochemical studies revealed that 3D scaffold indeed improved the viability of transplanted cells, their ability to incorporate into the in vivo neural circuit, and their capacity for tissue repair. This study indicates that hADSCs would have great therapeutic application potential as seeding cells for in vivo transplantation to treat various neurological diseases when co-applied with porous chitosan/gelatin bioscaffolds.
Figures


References
-
- Zuk P.A, Zuh M., Mizuno H., Huang J., Futrell J.W., Katz A.J., et al. . Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7,211, 2001 - PubMed
-
- Gronthos S., Franklin D.M., Leddy H.A., Robey P.G., Storms R.W., and Gimble J.M.Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 189,54, 2001 - PubMed
-
- Zhu Y., Liu T., Song K., Fan X., Ma X., and Cui Z.Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct 26,664, 2008 - PubMed
-
- Denker A.E., Nicoll S.B., and Tuan R.S.Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor beta-1. Differentiation 59,25, 1995 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

