Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression
- PMID: 24251624
- PMCID: PMC3965617
- DOI: 10.1111/jnc.12611
Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression
Abstract
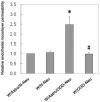
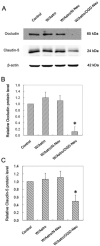
Blood-brain barrier (BBB) disruption occurring within the first few hours of ischemic stroke onset is closely associated with hemorrhagic transformation following thrombolytic therapy. However, the mechanism of this acute BBB disruption remains unclear. In the neurovascular unit, neurons do not have direct contact with the endothelial barrier; however, they are highly sensitive and vulnerable to ischemic injury, and may act as the initiator for disrupting BBB when cerebral ischemia occurs. Herein, we employed oxygen-glucose deprivation (OGD) and an in vitro BBB system consisting of brain microvascular cells and astrocytes to test this hypothesis. Neurons (CATH.a cells) were exposed to OGD for 3-h before co-culturing with endothelial monolayer (bEnd 3 cells), or endothelial cells plus astrocytes (C8-D1A cells). Incubation of OGD-treated neurons with endothelial monolayer alone did not increase endothelial permeability. However, when astrocytes were present, the endothelial permeability was significantly increased, which was accompanied by loss of occludin and claudin-5 proteins as well as increased vascular endothelial growth factor (VEGF) secretion into the conditioned medium. Importantly, all these changes were abolished when VEGF was knocked down in astrocytes by siRNA. Our findings suggest that ischemic neurons activate astrocytes to increase VEGF production, which in turn induces endothelial barrier disruption.
Keywords: VEGF; astrocyte; blood-brain barrier; neuron; oxygen-glucose deprivation.
© 2013 International Society for Neurochemistry.
Figures





Similar articles
-
Neutralization of interleukin-9 ameliorates experimental stroke by repairing the blood-brain barrier via down-regulation of astrocyte-derived vascular endothelial growth factor-A.FASEB J. 2019 Mar;33(3):4376-4387. doi: 10.1096/fj.201801595RR. Epub 2019 Jan 29. FASEB J. 2019. PMID: 30694693
-
Small GTPase RhoA and its effector rho kinase mediate oxygen glucose deprivation-evoked in vitro cerebral barrier dysfunction.Stroke. 2010 Sep;41(9):2056-63. doi: 10.1161/STROKEAHA.109.574939. Epub 2010 Jul 22. Stroke. 2010. PMID: 20651275
-
Effects of oxygen-glucose deprivation (OGD) on barrier properties and mRNA transcript levels of selected marker proteins in brain endothelial cells/astrocyte co-cultures.PLoS One. 2019 Aug 19;14(8):e0221103. doi: 10.1371/journal.pone.0221103. eCollection 2019. PLoS One. 2019. PMID: 31425564 Free PMC article.
-
The Translational Significance of the Neurovascular Unit.J Biol Chem. 2017 Jan 20;292(3):762-770. doi: 10.1074/jbc.R116.760215. Epub 2016 Dec 5. J Biol Chem. 2017. PMID: 27920202 Free PMC article. Review.
-
Role of TRP ion channels in cerebral circulation and neurovascular communication.Neurosci Lett. 2021 Nov 20;765:136258. doi: 10.1016/j.neulet.2021.136258. Epub 2021 Sep 22. Neurosci Lett. 2021. PMID: 34560190 Free PMC article. Review.
Cited by
-
Pathogenesis of brain edema and investigation into anti-edema drugs.Int J Mol Sci. 2015 Apr 30;16(5):9949-75. doi: 10.3390/ijms16059949. Int J Mol Sci. 2015. PMID: 25941935 Free PMC article. Review.
-
A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells.Front Cell Neurosci. 2019 Jun 6;13:230. doi: 10.3389/fncel.2019.00230. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31244605 Free PMC article.
-
Inhibition of MicroRNA-155 Supports Endothelial Tight Junction Integrity Following Oxygen-Glucose Deprivation.J Am Heart Assoc. 2018 Jun 26;7(13):e009244. doi: 10.1161/JAHA.118.009244. J Am Heart Assoc. 2018. PMID: 29945912 Free PMC article.
-
The Role of Gut Microbiota in Blood-Brain Barrier Disruption after Stroke.Mol Neurobiol. 2024 Dec;61(12):9735-9755. doi: 10.1007/s12035-023-03512-7. Epub 2023 Jul 27. Mol Neurobiol. 2024. PMID: 37498481 Review.
-
The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke.J Neuroinflammation. 2019 Nov 28;16(1):242. doi: 10.1186/s12974-019-1638-6. J Neuroinflammation. 2019. PMID: 31779652 Free PMC article.
References
-
- Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. - PubMed
-
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. - PubMed
-
- Bickler PE, Donohoe PH. Adaptive responses of vertebrate neurons to hypoxia. J Exp Biol. 2002;205:3579–3586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

