Synthetic aminoglycosides efficiently suppress cystic fibrosis transmembrane conductance regulator nonsense mutations and are enhanced by ivacaftor
- PMID: 24251786
- PMCID: PMC4068923
- DOI: 10.1165/rcmb.2013-0282OC
Synthetic aminoglycosides efficiently suppress cystic fibrosis transmembrane conductance regulator nonsense mutations and are enhanced by ivacaftor
Abstract
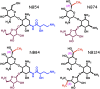
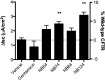
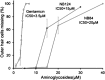
New drugs are needed to enhance premature termination codon (PTC) suppression to treat the underlying cause of cystic fibrosis (CF) and other diseases caused by nonsense mutations. We tested new synthetic aminoglycoside derivatives expressly developed for PTC suppression in a series of complementary CF models. Using a dual-luciferase reporter system containing the four most prevalent CF transmembrane conductance regulator (CFTR) nonsense mutations (G542X, R553X, R1162X, and W1282X) within their local sequence contexts (the three codons on either side of the PTC), we found that NB124 promoted the most readthrough of G542X, R1162X, and W1282X PTCs. NB124 also restored full-length CFTR expression and chloride transport in Fischer rat thyroid cells stably transduced with a CFTR-G542XcDNA transgene, and was superior to gentamicin and other aminoglycosides tested. NB124 restored CFTR function to roughly 7% of wild-type activity in primary human bronchial epithelial (HBE) CF cells (G542X/delF508), a highly relevant preclinical model with endogenous CFTR expression. Efficacy was further enhanced by addition of the CFTR potentiator, ivacaftor (VX-770), to airway cells expressing CFTR PTCs. NB124 treatment rescued CFTR function in a CF mouse model expressing a human CFTR-G542X transgene; efficacy was superior to gentamicin and exhibited favorable pharmacokinetic properties, suggesting that in vitro results translated to clinical benefit in vivo. NB124 was also less cytotoxic than gentamicin in a tissue-based model for ototoxicity. These results provide evidence that NB124 and other synthetic aminoglycosides provide a 10-fold improvement in therapeutic index over gentamicin and other first-generation aminoglycosides, providing a promising treatment for a wide array of CFTR nonsense mutations.
Figures




References
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. - PubMed
-
- Kerem E, Corey M, Kerem BS, Rommens J, Markiewicz D, Levison H, Tsui LC, Durie P. The relation between genotype and phenotype in cystic fibrosis—analysis of the most common mutation (delta F508) N Engl J Med. 1990;323:1517–1522. - PubMed
-
- Bedwell DM, Kaenjak A, Benos DJ, Bebok Z, Bubien JK, Hong J, Tousson A, Clancy JP, Sorscher EJ. Suppression of a CFTR premature stop mutation in a bronchial epithelial cell line. Nat Med. 1997;3:1280–1284. - PubMed
-
- Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med. 1996;2:467–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

