Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors
- PMID: 24251790
- PMCID: PMC4117552
- DOI: 10.1111/jcmm.12156
Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors
Abstract
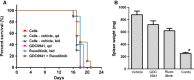
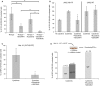
Current JAK2 inhibitors used for myeloproliferative neoplasms (MPN) treatment are not specific enough to selectively suppress aberrant JAK2 signalling and preserve physiological JAK2 signalling. We tested whether combining a JAK2 inhibitor with a series of serine threonine kinase inhibitors, targeting nine signalling pathways and already used in clinical trials, synergized in inhibiting growth of haematopoietic cells expressing mutant and wild-type forms of JAK2 (V617F) or thrombopoietin receptor (W515L). Out of 15 kinase inhibitors, the ZSTK474 phosphatydylinositol-3'-kinase (PI3K) inhibitor molecule showed strong synergic inhibition by Chou and Talalay analysis with JAK2 and JAK2/JAK1 inhibitors. Other pan-class I, but not gamma or delta specific PI3K inhibitors, also synergized with JAK2 inhibitors. Synergy was not observed in Bcr-Abl transformed cells. The best JAK2/JAK1 and PI3K inhibitor combination pair (ruxolitinib and GDC0941) reduces spleen weight in nude mice inoculated with Ba/F3 cells expressing TpoR and JAK2 V617F. It also exerted strong inhibitory effects on erythropoietin-independent erythroid colonies from MPN patients and JAK2 V617F knock-in mice, where at certain doses, a preferential inhibition of JAK2 V617F mutated progenitors was detected. Our data support the use of a combination of JAK2 and pan-class I PI3K inhibitors in the treatment of MPNs.
Keywords: JAK2; PI3K; combination treatment; kinases; myeloproliferative neoplasms.
© 2013 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures




References
-
- James C, Ugo V, Couedic Le JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. - PubMed
-
- Levine RL, Pardanani A, Tefferi A, et al. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

