New and emerging immune-targeted drugs for the treatment of multiple sclerosis
- PMID: 24251808
- PMCID: PMC4168378
- DOI: 10.1111/bcp.12285
New and emerging immune-targeted drugs for the treatment of multiple sclerosis
Abstract
Multiple sclerosis (MS) is a neurodegenerative disease with a major inflammatory component that constitutes the most common progressive and disabling neurological condition in young adults. Injectable immunomodulatory medicines such as interferon drugs and glatiramer acetate have dominated the MS market for over the past two decades but this situation is set to change. This is because of: (i) patent expirations, (ii) the introduction of natalizumab, which targets the interaction between leukocytes and the blood-CNS barrier, (iii) the launch of three oral immunomodulatory drugs (fingolimod, dimethyl fumarate and teriflunomide), with another (laquinimod) under regulatory review and (iv) a number of immunomodulatory monoclonal antibodies (alemtuzumab, daclizumab and ocrelizumab) about to enter the market. Current and emerging medicines are reviewed and their impact on people with MS considered.
Keywords: clinical trials; disease-modifying drugs; drug development; immunomodulatory drugs; monoclonal antibodies; neuroinflammation.
© 2013 The British Pharmacological Society.
Figures
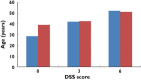
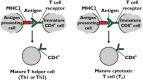
Similar articles
-
Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies.Mayo Clin Proc. 2014 Feb;89(2):225-40. doi: 10.1016/j.mayocp.2013.11.002. Mayo Clin Proc. 2014. PMID: 24485135 Review.
-
Disease-modifying agents in multiple sclerosis.Handb Clin Neurol. 2014;122:465-501. doi: 10.1016/B978-0-444-52001-2.00021-2. Handb Clin Neurol. 2014. PMID: 24507532 Review.
-
Established and novel disease-modifying treatments in multiple sclerosis.J Intern Med. 2014 Apr;275(4):350-63. doi: 10.1111/joim.12203. Epub 2014 Mar 11. J Intern Med. 2014. PMID: 24444048 Review.
-
New FDA-Approved Disease-Modifying Therapies for Multiple Sclerosis.Clin Ther. 2015 Apr 1;37(4):691-715. doi: 10.1016/j.clinthera.2015.03.001. Epub 2015 Apr 4. Clin Ther. 2015. PMID: 25846320 Review.
-
Drugs in development for relapsing multiple sclerosis.Drugs. 2013 May;73(7):625-50. doi: 10.1007/s40265-013-0030-6. Drugs. 2013. PMID: 23609782 Review.
Cited by
-
Alleviating nonlinear behavior of disulfide isoforms in the reversed-phase liquid chromatography of IgG2.J Chromatogr A. 2015 Sep 4;1410:147-53. doi: 10.1016/j.chroma.2015.07.098. Epub 2015 Aug 4. J Chromatogr A. 2015. PMID: 26256919 Free PMC article.
-
Lithium Chloride can Induce Differentiation of Human Immortalized RenVm Cells into Dopaminergic Neurons.Avicenna J Med Biotechnol. 2017 Oct-Dec;9(4):176-180. Avicenna J Med Biotechnol. 2017. PMID: 29090066 Free PMC article.
-
An integrin axis induces IFN-β production in plasmacytoid dendritic cells.J Cell Biol. 2022 Sep 5;221(9):e202102055. doi: 10.1083/jcb.202102055. Epub 2022 Jul 25. J Cell Biol. 2022. PMID: 35878016 Free PMC article.
References
-
- Bol Y, Smolders J, Duits A, Lange IM, Romberg-Camps M, Hupperts R. Fatigue and heat sensitivity in patients with multiple sclerosis. Acta Neurol Scand. 2012;126:384–389. - PubMed
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. - PubMed
-
- Palmer A. Pharmacotherapeuetic options for the treatment of multiple sclerosis. Clin Med Insights. 2012;4:1–24.
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. - PubMed
-
- WHO. Atlas Multiple Sclerosis. Geneva: WHO Press; 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

