Motor protein function in skeletal abdominal muscle of cachectic cancer patients
- PMID: 24251822
- PMCID: PMC3916119
- DOI: 10.1111/jcmm.12165
Motor protein function in skeletal abdominal muscle of cachectic cancer patients
Abstract
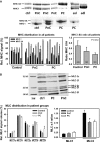
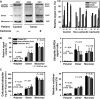
Cachexia presents with ongoing muscle wasting, altering quality of life in cancer patients. Cachexia is a limiting prognostic factor for patient survival and health care costs. Although animal models and human trials have shown mechanisms of motorprotein proteolysis, not much is known about intrinsic changes of muscle functionality in cancer patients suffering from muscle cachexia, and deeper insights into cachexia pathology in humans are needed. To address this question, rectus abdominis muscle samples were collected from several surgical control, non-cachectic and cachectic cancer patients and processed for skinned fibre biomechanics, molecular in vitro motility assays, myosin isoform protein compositions and quantitative ubiquitin polymer protein analysis. In pre-cachectic and cachectic cancer patient samples, maximum force was significantly compromised compared with controls, but showed an unexpected increase in myofibrillar Ca(2+) sensitivity consistent with a shift from slow to fast myosin isoform expression seen in SDS-PAGE analysis and in vitro motility assays. Force deficit was specific for 'cancer', but not linked to presence of cachexia. Interestingly, quantitative ubiquitin immunoassays revealed no major changes in static ubiquitin polymer protein profiles, whether cachexia was present or not and were shown to mirror profiles in control patients. Our study on muscle function in cachectic patients shows that abdominal wall skeletal muscle in cancer cachexia shows signs of weakness that can be partially attributed to intrinsic changes to contractile motorprotein function. On protein levels, static ubiquitin polymeric distributions were unaltered, pointing towards evenly up-regulated ubiquitin protein turnover with respect to ubiquitin conjugation, proteasome degradation and de-ubiquitination.
Keywords: cancer; contractility; motility assay; muscle cachexia; myosin isoforms.
© 2013 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


References
-
- Tisdale MJ. Mechanisms of cancer cachexia. Phys Rev. 2009;89:381–410. - PubMed
-
- Fortunati N, Manti R, Birocco N, et al. Pro-inflammatory cytokines and oxidative stress/antioxidant parameters characterize the bio-humoral profile of early cachexia in lung cancer patients. Oncol Rep. 2007;18:1521–7. - PubMed
-
- Cai D, Frantz JD, Tawa NE, Jr, et al. IKKbeta/NFkappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. - PubMed
-
- Bachmann J, Heiligensetzer M, Krakowski-Roosen H, et al. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg. 2008;12:1193–201. - PubMed
-
- Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

