Pharmacokinetic-pharmacodynamic modelling in anaesthesia
- PMID: 24251846
- PMCID: PMC4294078
- DOI: 10.1111/bcp.12286
Pharmacokinetic-pharmacodynamic modelling in anaesthesia
Abstract
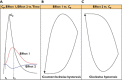
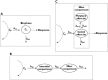
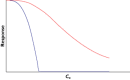
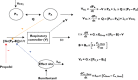
Anaesthesiologists adjust drug dosing, administration system and kind of drug to the characteristics of the patient. They then observe the expected response and adjust dosing to the specific requirements according to the difference between observed response, expected response and the context of the surgery and the patient. The approach above can be achieved because on one hand quantification technology has made significant advances allowing the anaesthesiologist to measure almost any effect by using noninvasive, continuous measuring systems. On the other the knowledge on the relations between dosing, concentration, biophase dynamics and effect as well as detection of variability sources has been achieved as being the benchmark specialty for pharmacokinetic-pharmacodynamic (PKPD) modelling. The aim of the review is to revisit the most common PKPD models applied in the field of anaesthesia (i.e. effect compartmental, turnover, drug-receptor binding and drug interaction models) through representative examples. The effect compartmental model has been widely used in this field and there are multiple applications and examples. The use of turnover models has been limited mainly to describe respiratory effects. Similarly, cases in which the dissociation process of the drug-receptor complex is slow compared with other processes relevant to the time course of the anaesthetic effect are not frequent in anaesthesia, where in addition to a rapid onset, a fast offset of the response is required. With respect to the characterization of PD drug interactions different response surface models are discussed. Relevant applications that have changed the way modern anaesthesia is practiced are also provided.
Keywords: PKPD models; anaesthesia; clinical applications; pharmacometrics.
© 2013 The British Pharmacological Society.
Figures
References
-
- Sheiner LB, Stanski DR, Vozeh S, Miller RD. Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–371. - PubMed
-
- Bouillon T, Schmidt C, Garstka G, Heimbach D, Stafforst D, Schwilden H. Hoeft A. Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of alfentanil. Anesthesiology. 1999;91:144–155. - PubMed
-
- Minto CF, Schnider TW, Short TG, Gregg KM, Gentilini A, Shafer SL. Response surface model for anesthetic drug interactions. Anesthesiology. 2000;92:1603–1616. - PubMed
-
- Borrat X, Trocóniz IF, Valencia JF, Rivadulla S, Sendino O, Llach J, Muñoz J, Castellví-Bel S, Jospin M, Jensen EW, Castells A, Gambús PL. Modeling the influence of the A118G polymorphism in the OPRM1 gene and of noxious stimulation on the synergistic relation between propofol and remifentanil: sedation and analgesia in endoscopic procedures. Anesthesiology. 2013;118:1395–1407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

