Epigallocatechin-3-gallate-mediated cardioprotection by Akt/GSK-3β/caveolin signalling in H9c2 rat cardiomyoblasts
- PMID: 24251870
- PMCID: PMC3871020
- DOI: 10.1186/1423-0127-20-86
Epigallocatechin-3-gallate-mediated cardioprotection by Akt/GSK-3β/caveolin signalling in H9c2 rat cardiomyoblasts
Abstract
Background: Epigallocatechin-3-gallate (EGCg) with its potent anti-oxidative capabilities is known for its beneficial effects ameliorating oxidative injury to cardiac cells. Although studies have provided convincing evidence to support the cardioprotective effects of EGCg, it remains unclear whether EGCg affect trans-membrane signalling in cardiac cells. Here, we have demonstrated the potential mechanism for cardioprotection of EGCg against H2O2-induced oxidative stress in H9c2 cardiomyoblasts.
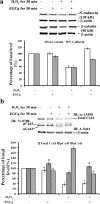
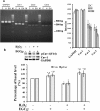
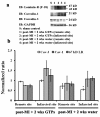
Results: Exposing H9c2 cells to H2O2 suppressed cell viability and altered the expression of adherens and gap junction proteins with increased levels of intracellular reactive oxygen species and cytosolic Ca2+. These detrimental effects were attenuated by pre-treating cells with EGCg for 30 min. EGCg also attenuated H2O2-mediated cell cycle arrest at the G1-S phase through the glycogen synthase kinase-3β (GSK-3β)/β-catenin/cyclin D1 signalling pathway. To determine how EGCg targets H9c2 cells, enhanced green fluorescence protein (EGFP) was ectopically expressed in these cells. EGFP-emission fluorescence spectroscopy revealed that EGCg induced dose-dependent fluorescence changes in EGFP expressing cells, suggesting that EGCg signalling events might trigger proximity changes of EGFP expressed in these cells. Proteomics studies showed that EGFP formed complexes with the 67 kD laminin receptor, caveolin-1 and -3, β-actin, myosin 9, vimentin in EGFP expressing cells. Using in vitro oxidative stress and in vivo myocardial ischemia models, we also demonstrated the involvement of caveolin in EGCg-mediated cardioprotection. In addition, EGCg-mediated caveolin-1 activation was found to be modulated by Akt/GSK-3β signalling in H2O2-induced H9c2 cell injury.
Conclusions: Our data suggest that caveolin serves as a membrane raft that may help mediate cardioprotective EGCg transmembrane signalling.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

