Intrinsic regulation of thyroid function by thyroglobulin
- PMID: 24251883
- PMCID: PMC3993028
- DOI: 10.1089/thy.2013.0344
Intrinsic regulation of thyroid function by thyroglobulin
Abstract
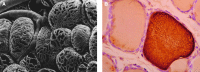
Background: The established paradigm for thyroglobulin (Tg) function is that of a high molecular weight precursor of the much smaller thyroid hormones, triiodothyronine (T3) and thyroxine (T4). However, speculation regarding the cause of the functional and morphologic heterogeneity of the follicles that make up the thyroid gland has given rise to the proposition that Tg is not only a precursor of thyroid hormones, but that it also functions as an important signal molecule in regulating thyroid hormone biosynthesis.
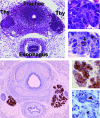
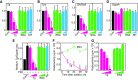
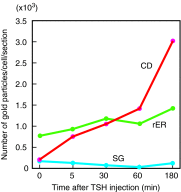
Summary: Evidence supporting this alternative paradigm of Tg function, including the up- or downregulation by colloidal Tg of the transcription of Tg, iodide transporters, and enzymes employed in Tg iodination, and also the effects of Tg on the proliferation of thyroid and nonthyroid cells, is examined in the present review. Also discussed in detail are potential mechanisms of Tg signaling in follicular cells.
Conclusions: Finally, we propose a mechanism, based on experimental observations of Tg effects on thyroid cell behavior, that could account for the phenomenon of follicular heterogeneity as a highly regulated cycle of increasing and decreasing colloidal Tg concentration that functions to optimize thyroid hormone production through the transcriptional activation or suppression of specific genes.
Figures

References
-
- Dunn JT, Dunn AD.2000Thyroglobulin: chemistry, biosynthesis, and proteolysis. In: Braverman LE, Utiger RD. (eds) Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. Eighth edition. Lippincott Williams & Wilkins, Philadelphia, PA, pp. 91–104
-
- van de Graaf SA, Ris-Stalpers C, Pauws E, Mendive FM, Targovnik HM, de Vijlder JJ.2001Up to date with human thyroglobulin. J Endocrinol 170:307–321 - PubMed
-
- Capen CC.2000Anatomy In: Braverman LE, Utiger RD. (eds) Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. Eighth edition. Lippincott Williams & Wilkins, Philadelphia, PA, pp 20–51
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

