Impact of the reduction of calcineurin inhibitors on renal function in heart transplant patients: a systematic review and meta-analysis
- PMID: 24251918
- PMCID: PMC4168377
- DOI: 10.1111/bcp.12289
Impact of the reduction of calcineurin inhibitors on renal function in heart transplant patients: a systematic review and meta-analysis
Abstract
Aims: Calcineurin inhibitors (CNIs) taken after heart transplantation lead to excellent short-term outcomes, but long-term use may cause chronic nephrotoxicity. Our aim was to identify, appraise, select and analyse all high-quality research evidence relevant to the question of the clinical impact of CNI-sparing strategies in heart transplant patients.
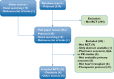
Methods: We carried out a systematic review and meta-analysis of randomized controlled trials on CNI reduction in heart transplant recipients. Primary outcomes were kidney function and acute rejection after 1 year. Secondary outcomes included graft loss, all-cause mortality and adverse events.
Results: Eight open-label studies were included, with 723 patients (four tested de novo CNI reduction and four maintenance CNI reduction). Calcineurin inhibitor reduction did not improve creatinine clearance at 12 months 5.46 [-1.17, 12.03] P = 0.32 I(2) = 65.4%. Acute rejection at 12 months (55/360 vs. 52/332), mortality (18/301 vs. 15/270) and adverse event rates (55/294 vs. 52/281) did not differ between the low-CNI and standard-CNI groups. There was significant benefit on creatinine clearance in patients with impaired renal function at 6 months [+12.23 (+5.26, +18.82) ml min(-1) , P = 0.0003] and at 12 months 4.63 [-4.55, 13.82] P = 0.32 I(2) = 75%.
Conclusions: This meta-analysis did not demonstrate a favourable effect of CNI reduction on kidney function, but there was no increase in acute rejection. To provide a better analysis of the influence of CNI reduction patterns and associated treatments, a meta-analysis of individual patient data should be performed.
Keywords: calcineurin inhibitor; heart transplantation; meta-analysis; renal function.
© 2013 The British Pharmacological Society.
Figures


References
-
- John R, Rajasinghe HA, Chen JM, Weinberg AD, Sinha P, Mancini DM, Naka Y, Oz MC, Smith CR, Rose EA, Edwards NM. Long-term outcomes after cardiac transplantation: an experience based on different eras of immunosuppressive therapy. Ann Thorac Surg. 2001;72:440–449. - PubMed
-
- Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. - PubMed
-
- Ekberg H, Bernasconi C, Tedesco-Silva H, Vitko S, Hugo C, Demirbas A, Acevedo RR, Grinyo J, Frei U, Vanrenterghem Y, Daloze P, Halloran P. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9:1876–1885. - PubMed
-
- Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, Mulgaonkar S, Meier-Kriesche HU, Patel D, Bloom RD. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the Opticept trial. Am J Transplant. 2009;9:1607–1619. - PubMed
-
- Kobashigawa J, Miller L, Renlund D, Mentzer R, Alderman E, Bourge R, Costanzo M, Eisen H, Dureau G, Ratkovec R, Hummel M, Ipe D, Johnson J, Keogh A, Mamelok R, Mancini D, Smart F, Valantine H. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation. 1998;66:507–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

